Comparison of mammosphere formation from breast cancer cell lines and primary breast tumors
Introduction
Recent years, increasing evidence indicates that cancer originates from a small fraction of tumor initiating cells with the abilities of self-renewal, unlimited propagation, multipotent differentiation and giving rise to phenotypically distinct cells found within the tumor population. Such capacities share similarity with normal stem cells. Thus, these cells are also called cancer stem cells (CSCs) (1,2). The existence of CSCs had been successfully approved in variety of tumors such as leukemia, breast cancer, prostate cancer, ovarian cancer, glioma, and gastrointestinal cancer, and had been successfully isolated and cultured in vitro (2,3). CSCs are resistant to standard chemotherapy and radiotherapy (4,5). It is believed that CSCs are not only the source of the tumor, but also may be responsible for tumor progression, metastasis, resistance to therapy, and subsequent tumor recurrence. Therefore, a better understanding of the biology of CSCs in each tumor may be a critical step toward the development of treatments to eventual cure of cancer (6,7).
Breast cancer stem cells (BCSCs) were first identified by Al-Hajj and colleagues (3). They inoculated human breast cancer cells to the mammary fat pad of severe combined immunodeficiency disease (SCID) mice, and found that only a minority of breast cancer cells had the ability to form new tumors. These cells were CD44+/CD24–/low/lineage–. Dontu and colleagues (8) developed an in vitro culture system that allows for propagation of human mammary epithelial cells (HMECs) in non-adherent non-differentiated culture conditions. Cells capable of surviving and proliferating in such conditions formed discrete clusters of cells termed “mammospheres”. Such spheroids were enriched in progenitor cells capable of differentiating along multiple lineages including luminal, myoepithelial and alveolar. Ponti and colleagues (9) found that 95% to 96% of cells in mammospheres cultured from cell lines and primary breast tumors were CD44+/CD24–/low.
Generally stated, BCSCs can be isolated or enriched by sorting breast cancer cells for CD44+/CD24–/low cells by selection for side-population (10), or by culturing of cells in non-adherent non-differentiating conditions to form mammospheres (8,9). In breast cancer, the mammosphere culture system has been widely used to identify and enrich for putative CSCs from breast cancer cell lines or primary breast tumors. Serum-free culture has been proven to be an efficient way to enrich tumor stem cells, but culturing mammospheres from primary breast tumors still remains an obstacle to many researchers (11).
Use of primary breast tumor cells is considered to be the best means to study tumor repopulation (12). However, experiments with primary tumor cells are costly and difficult to control because of the heterogeneous nature of the cellular, genetic, and epigenetic composition among patient tissue samples. In order to overcome problems associated with using primary human tissues, continuous breast cancer cell lines have been developed from various sources. Despite their acquired ability to grow in vitro, cell lines continue to share many of the molecular and genetic features of the primary breast cancers from which they were derived. In this study, a luminal subtype cell line MCF-7 and a basal subtype cell line MDA-MB-231 both derived from pleural effusion of breast cancer patients (13-16) were chosen. We explored the optimal culturing system for BCSCs from breast cancer cell lines and primary breast tumors. Then, the mammosphere formation efficiency (MFE), the CD44+/CD24–/lowESA+Lin– cell proportion in mammospheres, and the tumorigenecity of mammospheres generated from the two breast cancer cell lines and primary breast tumors were compared. This study validates the use of breast cancer cell lines as models to elucidate the nature of BCSCs.
Materials and methods
Culture of MCF-7 and MDA-MB-231 cell lines
Human breast epithelial adenocarcinoma cells MCF-7 and MDA-MB-231 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). As monolayer culture, cells were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS, Hyclone), 100 units/mL penicillin and 100 µg/mL streptomycin (Invitrogen, Carlsbad, CA) at 37 °C in a humidified atmosphere with 5% CO2.
Mammosphere culture from cell lines
For mammosphere culture from MCF-7 and MDA-MB-231 cells, cells were suspended at 1×105 cells/mL and seeded into ultralow attachment plates (Corning, NY, USA) in serum free DMEM/F12 (1:1) supplemented with 10 ng/mL basic fibroblast growth factor (b-FGF, peprotech, St. Louis, MO, USA), 20 ng/mL epidermal growth factor (EGF, peprotech), ITS (insulin + transferrin + selenium, Sigma), with or without B27 (GIBCO). Two milliliters of fresh media was added to each well every two days (without removing the old media). Cells grown in these conditions as nonadherent spherical clusters of cells (usually named ‘‘mammospheres’’) were collected every seven days by gentle centrifugation and dissociated to single cell suspensions using the method of Dontu et al. (8).
Mammosphere culture from breast tumors
From April 2011 to December 2011, 39 fresh breast invasive ductal carcinoma tissue samples were obtained from the Department of Thyroid and Breast Surgery, West China Hospital, Sichuan University. All patients were female which did not receive any treatment before operation.
Mammosphere culture was performed according to the method of Dontu et al. (8) with modifications. After histologic assessment, the tumor lesions were sent to the laboratory within 30 mins of surgery. The tissue were disaggregated mechanically into pieces around 1 mm3 and digested enzymatically for 40-60 mins at 37 °C in a 1:1 solution of collagenase/hyaluronidase (Sigma). After filtration through a 70 µm pore filter, single cells were plated in DMEM/F-12 (Hyclone) supplemented with 10 ng/mL bFGF, 20 ng/mL EGF, ITS with our without B27, incubated in 37 °C incubator containing 5% CO2. Mammospheres were enzymatically dissociated every 7 days by incubation in a 0.5% trypsin-EDTA solution (Invitrogen) for about 5-10 mins at 37 °C and plated at 1×105 cells/mL in the growth media described above.
Mammosphere formation assays
Mammospheres from MCF-7, MDA-MB-231 cells and primary breast tumors were plated at 1×105 single cells/mL into ultralow attachment plates. The number of spheres (diameter >50 μm) for each well was evaluated under microscope on days 7, 14 and 21, respectively. MFE was calculated as the number of spheres divided by the original number of cells seeded and expressed as percentage means (± SD).
Flow cytometry
Mammoshperes from MCF-7, MDA-MB-231 cells and primary breast tumors were collected, washed with phosphate-buffered saline (PBS) and then enzymatically dissociated with 0.05% trypsin/0.25% EDTA into single cell suspension. Combinations of monoclonal antibodies against human CD44-PE, CD24-FITC (BD Biosciences, San Diego, CA, USA), lineage makers (CD2, CD3, CD10, CD16,CD18, CD31, CD64,CD140b-PE-cy7, all from Pharmingen) and ESA-PE-Cy 5.5 (BD) were added to the cell suspension at concentrations recommended by the manufacturer and incubated at 4 °C in dark for 30 to 40 mins. For all mammospheres, labeled cells were washed in PBS to eliminate unbounded antibody, and then analyzed on a FACSAria (MountainView, CA, USA).
Tumorigenicity assay
Nude mice purchased from Beijing HFK Bioscience CO were maintained in laminar flow rooms under constant temperature and humidity. Mammospheres were collected, enzymatically dissociated, washed in PBS, and kept at 4 °C until 1,000 cells were suspended in 50-100 μL matrigel and slowly injected into mammary fat pad of 6-week-old female nude mice. Mice were inspected for tumor formation by observation and palpation for 10 weeks after injection. After this time interval, all mice were sacrificed by cervical dislocation. At sacrifice, the xenograft tumors were immediately removed, fixed in 10% neutral buffered formalin solution (Sigma), and embedded in paraffin.
Statistical analysis
The results are presented as the mean ± SD for at least three individual. P values of 0.05 or less, calculated using a paired two-sided Student’s t-test were considered to indicate statistically significant differences.
Results
Optimize mammosphere culture system
Serum-free culture has been proven to be an efficient way to enrich tumor stem cells, but culturing mammospheres from primary breast tumors still remain difficult to many researchers (11). We carried out a series of exploration on culture conditions, such as different digestion time and medium composition.
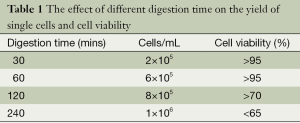
For BCSCs primary culture from breast tumors, collagenase/hyaluronidase (1:1) is used for digestion of tumor tissue into single cells. In order to get enough number of cells with optimal cell viability, we tried different digestion time (Table 1). We found out digestion time around 60 mins gave rise to enough single cells with the highest cell viability.
Full table
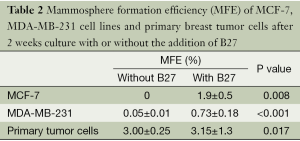
According to the literature (9), the culture medium for mammosphere contains serum-free DMEM/F12 (1:1) supplemented with 10 ng/mL b-FGF, 20 ng/mL EGF, 5 µg/mL insulin and selenium 0.4% bovine serum albumin. However, in our experiment, MCF-7 and MDA-MB-231 cells grown in this condition tended to attach to the dish and could hardly form spheres. B27 was used for culturing embryonic neurons (17), and also for mammosphere culturing (8,11). We added B27 as a supplement to promote mammosphere formation. With the addition of B27, MFE of both cell lines and primary breast tumor cells improved significantly (Table 2).
Full table
Thus, enzymatic digestion of 60 min and the addition of B27 to the culture medium were optimal for mammosphere culturing.
Mammosphere formation from cell lines and primary breast tumors
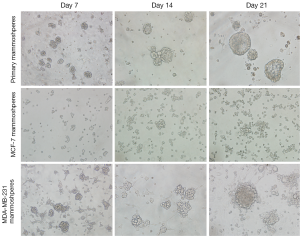
MCF-7 cells were capable of forming mammospheres of 50 µm in diameter under mammosphere culture condition for 2 weeks. After 3 weeks of culture, more mammospheres appeared and the diameter increased to around 100 µm. While in MDA-MB-231 cells and primary breast tumor cells, mammospheres appeared after 1 week culture under mammosphere culture condition. Cultured for 3 weeks, mammospheres enlarged to 100-200 µm in diameter (Figure 1). After three week culture, mammospheres from MDA-MB-231 cells tended to attach. Thus, both breast cancer cell lines and primary breast tumor cells were capable of forming mammospheres under mammosphere culture condition. However, mammospheres from different origins showed different morphology.
Mammosphere formation efficiency (MFE)
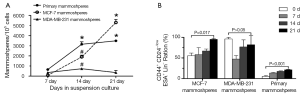
After one week culture under mammosphere culture condition, MFE of MCF-7, MDA-MB-231 cell lines, and primary breast tumors were 0.07%±0.036%, 0.356%±0.083%, and 0.654%±0.059%, respectively. After three week culture, MFE of the above cells increased to 5.30%±0.307%, 0.35%±0.204%, 3.492%±0.11%, respectively (Table 3, Figure 2A). Thus, MCF-7 had the highest MFE among these three cells.

Full table
Mammoshperes enriched for CD44+/CD24-/lowESA+Lin- cells
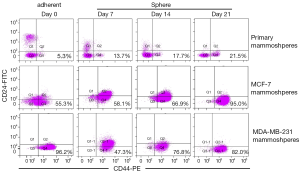
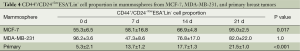
The proportion of CD44+/CD24–/lowESA+Lin– cells in the three mammospheres was detected at 0, 7, 14, 21 days after culture by flow cytometry (Figures 2B,3). Before culturing under mammosphere culture condition, proportion of CD44+/CD24–/lowESA+Lin– cells in MCF-7, MDA-MB-231 cell lines, and primary breast tumors were 55.3%±6.5%, 96.2%±3.6%, and 5.3%±2.1%, respectively. After 3 weeks culture under mammosphere culture condition, CD44+/CD24–/lowESA+Lin– cell proportion in mammospheres from MCF-7 cells and primary breast tumors significantly increased to 95.0%±2.5% and 21.5%±1.0%, respectively (Table 4, Figure 2B). However, CD44+/CD24–/lowESA+Lin– cell proportion in MDA-MB-231 cells didn’t increase under mammosphere culture condition. Thus, BCSCs can be enriched by culturing of MCF-7 cells and primary breast tumor cells in non-adherent non-differentiating conditions to form mammospheres.
Full table
Tumorigenicity of mammospheres from MCF-7, MDA-MB-231 cell lines, and primary breast tumors
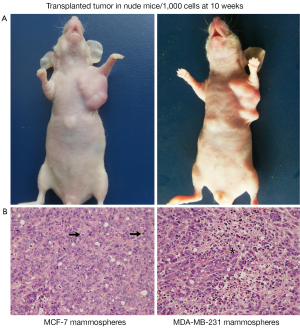
Ponti et al. (9) were the first to report that cells initiated from MCF-7 mammospheres could form tumors when as few as 1,000 cells were injected to SCID mice. Accordingly, in our study, Mammospheres from MCF-7, MDA-MB-231 cell lines, and primary breast tumors were enzymatically digested into single cells, 1,000 cells were injected into mammary fat pad of nude mice. After 4 weeks, cells from MCF-7 and MDA-MB-231 mammospheres could form palpable tumors in 3 of 4 and 4 of 4 nude mice, respectively. The tumors became obvious at 10 weeks (Figure 4A). However original MCF-7 and MDA-MB-231 cells and cells from primary mammosphere failed to form tumors. H&E staining of tumors grown in mice after injection of tumor initiating cells revealed the presence of malignant cells, with large nuclei and active mitosis; In some area, erythrocytes were visible within tumor cell lined cavities (Figure 4B). These data show that cells from MCF-7 and MDA-MB-231 mammospheres have tumor-initiating capacity in vivo, when compared with original MCF-7 and MDA-MB-231 cells.
Discussion
Although growing evidence has revealed the existence of CSC in a variety of human cancers (3,18-21). Culturing mammospheres from breast cancer cell lines and primary breast tumors still remains an obstacle to many researchers (11). In this study, we tried to optimize the culture condition of BCSCs from MCF-7 cells, MDA-MB-231 cells and primary breast tumors. Meanwhile, we compared their MFE, CD44+/CD24–/lowESA+Lin– cell proportion in mammopheres, and tumorigenicity.
For mammosphere culture from primary breast tumors, we found enzymatic digestion time around 60 min gave rise to enough single cells with the highest cell viability (Table 1). What we need to notice is to adjust the digestion time according to pathological characters of tumors. For example, breast adenocarcinoma is usually hard to digest. While, mucinous carcinoma is relatively fragile, which needs shorter digestion time.
Without the addition of B27, low MFE always presented in mammospheres from primary breast tumors. At the same time, mammospheres from breast cancer cell lines were easy to attach. Although B27 was initially used for the culture of progenitor or stem cells from central or peripheral nervous system (17), many researchers found B27 was an essential component of culture medium necessary for non-adherent cells suspension (11,22). With the addition of B27 to the culture medium, MFE was significantly increased (Table 2), owing to the role of B27 in increased survival of tumor spheres (8,23,24) and prevention of adherences (11). Thus, enzymatic digestion of 60 min and the addition of B27 to non-adherent non-differentiated culture medium were optimal for mammosphere culturing.
Mammospheres from different origins showed different morphology. Mammoshperes from primary breast tumors tended to develop into regular round shape. Whereas, mammoshperes from MCF-7 cells were relatively loose. Mammoshperes from MDA-MB-231 cells grew bigger than 200 µm in diameter, which tended to attach after 3-week culture (Figure 1).
Fillmore CM et al. reported basal type cell line MDA-MB-231 consisted more than 90% CD44+/CD24–/low cells (7). Moreover, cell lines with high CD44+/CD24– cell numbers express basal/mesenchymal or myoepithelial but not luminal markers (25). Consisted with these results, our data showed before culturing under mammosphere culture condition, proportion of CD44+/CD24–/lowESA+Lin– cells in MCF-7, MDA-MB-231 cell lines, and primary breast tumors were 55.3%±6.5%, 96.2%±3.6%, and 5.3%±2.1%, respectively. Rosen et al. reported more than 90% of cells in mammospheres cultured from MCF-7 cells and primary breast tumors had CD44+/CD24–/low/lineage– phenotype (26). After 3 weeks culture under mammosphere culture condition, we found CD44+/CD24–/lowESA+Lin– cell proportion in MCF-7 cells and primary breast tumors significantly increased to 95.0%±2.5% and 21.5%±1.0%, respectively (Table 4, Figure 2B). However, under mammosphere culture condition, CD44+/CD24–/lowESA+Lin– cell proportion in MDA-MB-231 cell line did not increase (Table 4, Figure 2B), probably due to the already high stem cell proportion in MDA-MB-231 cell line.
In our study, MFE of MCF-7 cells and primary breast tumors is higher than MDA-MB-231 cells (Table 3). Ponti D reported long-term cultures which could be expanded as floating mammospheres for more than 40 passages in vitro derived only from estrogen receptor—positive lesions and estrogen receptor—positive MCF-7 cells (9). These data indicates a correlation between estrogen receptor expression and mammosphere formation that needs to be further investigated.
Ponti et al. (9) reported that cells initiated from MCF-7 mammospheres could form tumors when as few as 1,000 cells were injected to SCID mice. Cell lines with high proportion of CD44+/CD24– subpopulation were more invasive than other cell lines (25). Our study found that 1,000 cells from MCF-7 and MDA-MB-231 mammospheres with high proportion of CD44+/CD24– cell proportion detected by flow cytometry could form tumors in nude mice in 4 weeks after subcutaneous injection. Nevertheless, the primary mammopheres and the two cell lines failed to form tumors. Primary mammospheres especially the first generation consists of cells of heterogenous origins, in which myoepithelial cells and luminal epithelial cells both could form mammospheres (8). The percentage of breast cancer stem cells was 68% in the first generation mammospheres, and increased to 98% in second and later generation mamospheres (8). Therefore, mammospheres from purified cell lines could be more tumorigenic than primary mammospheres.
Conclusions
Under non-adherent and non-differentiated culture condition, mammospheres could be formed in breast cancer cell lines MCF-7, MDA-MB-231, and primary breast tumors. MCF-7 cells showed highest MFE and stem cell proportion after three week culture. Cells from both MCF-7 and MDA-MB-231 mammospheres were tumorigenic. This study validates the use of breast cancer cell lines as well as primary breast tumors for the research of breast cancer stem cells.
Acknowledgements
The authors gratefully acknowledge insightful comments and expertise from Tie Chen (Laboratory of Stem Cell Biology). We also thank Shengliang Zhang for suggestions on drawing tools and with analytical validation. Also at Laboratory of Stem Cell Biology, thanks goes to Qiaorong Huang and Xue Li with providing support on flow cytometry, to Yuehe Fu for assistance with animal grooming and tissue handling.
Funding: This study was supported by the National Natural Science foundation of China (grant No. 81001176).
Disclosure: The authors declare no conflict of interest.
References
- Schulenburg A, Ulrich-Pur H, Thurnher D, et al. Neoplastic stem cells: a novel therapeutic target in clinical oncology. Cancer 2006;107:2512-20. [PubMed]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006;355:1253-61. [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [PubMed]
- Li X, Lewis MT, Huang J, et al. Intrinsic Resistance of Tumorigenic Breast Cancer Cells to Chemotherapy. J Natl Cancer Inst 2008;100:672-9. [PubMed]
- Phillips TM, McBride WH, Pajonk F. The Response of CD24−/low/CD44+ Breast Cancer–Initiating Cells to Radiation. J Natl Cancer Inst 2006;98:1777-85. [PubMed]
- Al-Hajj M, Becker MW, Wicha M, et al. Therapeutic implications of cancer stem cells. Current Opinion in Genetics Development 2004;14:43-7. [PubMed]
- Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 2008;10:R25. [PubMed]
- Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003;17:1253-70. [PubMed]
- Ponti D, Costa A, Zaffaroni N, et al. Isolation and In vitro Propagation of Tumorigenic Breast Cancer Cells with Stem/Progenitor Cell Properties. Cancer Res. 2005;65:5506-11. [PubMed]
- Patrawala L, Calhoun T, Schneider-Broussard R, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res 2005;65:6207-19. [PubMed]
- Huang MZ, Zhang FC, Zhang YY. Influence factors on the formation of mammospheres from breast cancer stem cells. Beijing Da Xue Xue Bao 2008;40:500-4. [PubMed]
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006;66:9339-44. [PubMed]
- Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006;10:515-27. [PubMed]
- Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat 2004;83:249-89. [PubMed]
- Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res 1997;57:3071-8. [PubMed]
- Subik K, Lee JF, Baxter L. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical Analysis in Breast Cancer Cell Lines. Breast Cancer (Auckl) 2010;4:35-41. [PubMed]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods 1997;71:143-55. [PubMed]
- Singh SK, Clarke ID, Hide T, et al. Cancer stem cells in nervous system tumors. Oncogene 2004;23:7267-73. [PubMed]
- Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 2005;65:9328-37. [PubMed]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106-10. [PubMed]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111-5. [PubMed]
- Chen H. The effect of B27 supplement on promoting in vitro propagation of Her2/neu-transformed mammary tumorspheres. J Biotech Res 2011;3:7-18.
- Svendsen CN, Fawcett JW, Bentlage C, et al. Increased survival of rat EGF-generated CNS precursor cells using B27 supplemented medium. Exp Brain Res 1995;102:407-14. [PubMed]
- Brewer GJ, Torricelli JR, Evege EK, et al. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res 1993;35:567-76. [PubMed]
- Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 2006;8:R59. [PubMed]
- Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science 2009;324:1670-3. [PubMed]