
In vivo experimental models account for higher complexity than in vitro preclinical settings in cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide. There is no current treatment able to efficiently treat the disease as the lung cancer is often diagnosed at an advanced stage. Furthermore, lung cancer cells are often resistant or acquire resistance to the treatment. There is an unprecedent need to understand the mechanisms driving lung tumorigenesis, aggressiveness, metastasization, and resistance to treatments as they could provide new tools for detecting the disease at an earlier stage and for a more tailored and successful therapy. KRAS mutations are the most frequent oncogenic aberrations in lung cancer patients. However, even if this oncogenic driver and its biology have been characterized since long time, so far, no therapy targeting hyperactivated KRAS has been developed to efficiently treat the disease (1). The reason for this is that Ras controls multiple oncogenic signalling, as well as apoptotic/senescence signalling as a negative feedback mechanism. In this scenario, targeting only one of those signallings does not reveal much effectiveness in clinics. Thus, it is important to extend the knowledge of Ras biology and to identify new pathways mediated by Ras or intersecting with Ras signalling, to find possible synthetic lethal partners for a more effective RAS targeted therapy. Ras association domain family 1 isoform A (RASSF1A) is a tumor suppressor found to be epigenetically inactivated in many tumor types, including human lung cancers (2-5). It physically associates with oncogenic KRAS, and mediates apoptosis and senescence thereby providing a feedback mechanism that opposes excessive oncogenic Ras activity. RASSF1A oncosuppressive function is elicited through multiple mechanisms. On one hand it functions as a scaffold protein for KRAS in the direct activation of pro-apototic pathways, on the other hand it indirectly modulates KRAS mediated pro-mitogenic signalling through the modulation of Ras mitogenic effectors (PI3K/AKT, MAPK/ERK, RALGEF) (6). RASSF1A also links RAS to the Hippo pathway, while RASSF1A loss uncouples Ras from this oncosuppressive pathway (7-10). Finally, it has been shown that RASSF1A attenuates pro-inflammatory events (11), even if the role of RASSF1A in the control of Ras-driven inflammation is yet unexplored. Lung cancer patients with activated oncogenic Ras and reduced RASSF1A expression exhibit poor prognosis (12). However, in vivo evidence of the oncosuppressive role of RASSF1A in a mutant KRAS context in lung is still scarce.
RASSF1 inactivation in vivo increases KRAS driven tumorigenesis through unidentified pathways that are independent on MAPK, RAL and YAP activation
Shmidt et al. originally analysed in vivo the mechanisms through which RASSF1A acts as a tumor suppressor in KRAS driven tumors. They elegantly report that in RAS12D+ mice, RASSF1A haploinsufficiency (which recapitulates its epigenetic silencing observed in human tumors) enhances the tumorigenicity of KRAS (measured as an increased number of tumor masses, with no apparent increase in tumor average size (Figure 1A, left and right panels) as well as the KRAS-related inflammation. Conversely, in tumoral and in non-tumoral tissues in which RAS is not hyperactivated, loss of RASSF1A only upregulates the RAS mediated mitogenic pathways (to the same fashion observed in mutant KRAS background) without any detectable effects on tumorigenesis (Figure 1B, left and right panels). The authors do not clarify what pathways are differentially affected in the KRAS wt vs. mutated background upon RASSF1A inactivation. Thus, the reported evidences do not provide any specific mechanism to explain the role of KRAS/RASSF1A axis in lung cancer.
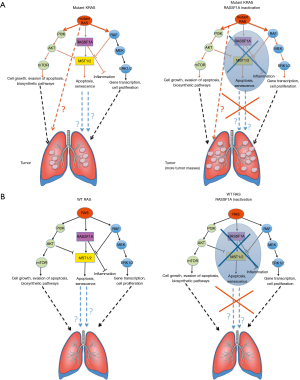
In particular, the authors do not describe any mechanism that can be responsible of lung tumorigenesis in KRAS mutated but not in KRAS wt background upon RASSF1A inactivation.
In detail, the authors report that the loss of RASSF1A determines:
- an increased expression of the oncogenic YAP in KRAS mutant tumors. In the same experimental setting expressing RASSF1A, YAP is maintained at lower levels. This is because RASSF1A mediates RAS-induced activation of the Hippo pathway as a feedback mechanism;
- an increase of the mitogenic AKT phosphorylation and MAPK activation;
- an increased expression of Ras related RAL oncoproteins;
- an augmented RAS-mediated inflammation as increased CD11 infiltration and IL-6 expression (this latter effect was observed both in murine system in vivo and in human cell lines in vitro).
However, the effects on YAP, AKT and RAL signalling occur both in KRAS wt and in mutated contexts, suggesting that these mitogenic pathways, at least in this experimental system, are not responsible for driving the increased tumorigenesis observed in the KRAS mutant background compared to its wt counterpart. This strongly suggests that KRAS hyperactivation and concomitant loss of RASSF1A trigger other pathways that need to be investigated in order to explain the different behaviour of KRAS mutated vs. KRAS wt lung tumor upon RASSF1A inactivation (Figure 1). Moreover, this provides further evidence that tumor biology in vivo is much more complex and cannot always be explained through the simple dissection of molecular mechanisms in vitro. Indeed, such complexity might have important implications in clinics. Based on this, the authors suggest that it is necessary to take into account the KRAS activation status of the lung tumor in order to define the best therapeutic approach. This reinforces the general emerging concept that precise information about the genetic and epigenetic landscape of each tumor is required to better predict the response to different targeted therapies and to design the best therapeutic options for each patient. Moreover, this work opens the road for new studies aimed at investigating what other pathways can be differentially affected in the KRAS wt or mutated background upon RASSF1A inactivation. This might lead to the identification of those signalling pathways responsible for a more in vivo aggressive phenotype as such due to RASSF1A inactivation in a KRAS mutated background.
Acknowledgements
Funding: Work in the author lab is supported by AIRC (IG: 20613).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roman M, Baraibar I, Lopez I, et al. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer 2018;17:33. [Crossref] [PubMed]
- Dammann R, Li C, Yoon JH, et al. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 2000;25:315-9. [Crossref] [PubMed]
- Agathanggelou A, Honorio S, Macartney DP, et al. Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene 2001;20:1509-18. [Crossref] [PubMed]
- Burbee DG, Forgacs E, Zochbauer-Muller S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst 2001;93:691-9. [Crossref] [PubMed]
- Endoh H, Yatabe Y, Shimizu S, et al. RASSF1A gene inactivation in non-small cell lung cancer and its clinical implication. Int J Cancer 2003;106:45-51. [Crossref] [PubMed]
- Donninger H, Schmidt ML, Mezzanotte J, et al. Ras signaling through RASSF proteins. Semin Cell Dev Biol 2016;58:86-95. [Crossref] [PubMed]
- Matallanas D, Romano D, Al-Mulla F, et al. Mutant K-Ras activation of the proapoptotic MST2 pathway is antagonized by wild-type K-Ras. Mol Cell 2011;44:893-906. [Crossref] [PubMed]
- Del Re DP, Matsuda T, Zhai P, et al. Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Invest 2010;120:3555-67. [Crossref] [PubMed]
- Romano D, Maccario H, Doherty C, et al. The differential effects of wild-type and mutated K-Ras on MST2 signaling are determined by K-Ras activation kinetics. Mol Cell Biol 2013;33:1859-68. [Crossref] [PubMed]
- Lo Sardo F, Strano S, Blandino G. YAP and TAZ in Lung Cancer: Oncogenic Role and Clinical Targeting. Cancers (Basel) 2018.10. [PubMed]
- Gordon M, El-Kalla M, Zhao Y, et al. The tumor suppressor gene, RASSF1A, is essential for protection against inflammation -induced injury. PLoS One 2013;8:e75483. [Crossref] [PubMed]
- Kim DH, Kim JS, Park JH, et al. Relationship of Ras association domain family 1 methylation and K-ras mutation in primary non-small cell lung cancer. Cancer Res 2003;63:6206-11. [PubMed]


