Clinical Practice Guidelines for Diagnosis and Management of Cough—Chinese Thoracic Society (CTS) Asthma Consortium
Introduction
Cough is the most common symptom in respiratory specialist clinics of tertiary hospitals and outpatient clinics of primary health care facilities. In China, patients with chronic cough account for at least one third of all patients referred to respiratory specialist clinics. Chronic cough without significant abnormal chest radiographic findings is often misdiagnosed as chronic bronchitis or chronic pharyngitis. Misdiagnosis of cough results in unnecessary repetitive testing, such as chest radiographs or computed tomography (CT), and widespread abuse of antibiotics or antitussives with little improvement, and potential adverse effects. Chronic cough impair quality of life badly cause severe economic burden in China (1-5).
To further standardize the diagnosis and treatment of acute and chronic cough, thus providing guidance for clinical practice, the Panel of Chinese Thoracic Society (CTS) Asthma Consortium released the first edition of the Chinese Guidelines for Diagnosis and Treatment of Cough (Draft) in 2005 (6). This document was updated in 2009 (7). The Chinese Cough Guidelines were established based on the evidence of clinical research, expert opinions, and recommendations from the cough guidelines endorsed by the American College of Chest Physicians (ACCP), European Respiratory Society (ERS), Japanese Respiratory Society etc. (8-11). Compared with these guidelines, the Chinese Cough Guidelines vary slightly in structure and content, according to clinical evidence and practice in China. Since the release of the Chinese Cough Guidelines, the management of cough in China has been improved. Recently, there have been significant advances in cough research and increased understanding of the pathogenesis, etiology, diagnosis, and management of cough. To further refine the guidelines and include the latest evidence, in 2014 the CTS Asthma Consortium initiated a task force to revise the 2009 Chinese Guidelines for Diagnosis and Management of Cough. For the first time, evidence-based methodology was adopted according to the requirements for guideline development in China. A comprehensive literature review was undertaken and recommendations were made. This updated revision updated or added the following sections: (I) introduction of evidence-based methodology for guideline development; (II) updated and expanded sections as compared to previous versions; (III) an additional section on the evaluation of cough; (IV) Traditional Chinese Medicine (TCM) for the management of cough was added; (V) the etiology and management of chronic cough in children was introduced; (VI) a section on uncommon causes of chronic cough; and (VII) added unexplained cough [refractory cough, cough hypersensitivity syndrome (CHS)].
Introduction of methodology
- The target population: patients with cough.
- The target users: respiratory specialists from all levels of hospitals, physicians of internal medicine and TCM, general practitioners, pediatricians, and other health-care providers.
- Members of the panel: specialists in respiratory medicine, ear-nose-throat, pediatrics, gastroenterology, and TCM; evidence-based medicine professionals, clinical epidemiologists, and medical editors.
- The search database included: (i) English databases: PubMed/Medline, Embase, and Cochrane Library; (ii) Chinese databases: China Biology Medicine disc (CBMdisc), Wanfang Data, China Academic Journals full-text database (CNKI), and Chongqing VIP (CQVIP). The literature search ended with papers published on June 30, 2015. Two independent groups conducted the literature search for each specific clinical issue according to the inclusion and exclusion criteria. An appraisal of the literature using a specifically designed form was performed. Respiratory physicians conducted the preliminary evaluation of the literature. In cases where consensus could not be obtained due to difficulty in literature appraisal, a meeting of the guideline panel was held for critical review and reappraisal. If necessary, the literature search and evaluation would be conducted again.
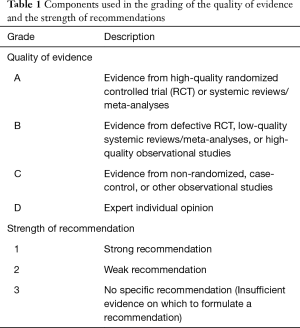
- Quality of evidence and grade of recommendation: The current guideline adopted a grading system for assessing quality of evidence and grading recommendation. The grading system is a combination of the grading system used in the American College of Chest Physicians (ACCP) Guidelines for Diagnosis and Management of Cough [2006] (8,12) and GRADE (grading of recommendations assessment, development, and evaluation) (13) (Table 1). The level of evidence was graded in four categories: high, moderate, low, and very low, and presented as A, B, C, and D, respectively. The strength of recommendation was: 1, strong; 2, weak; and 3, no specific recommendation. The quality assessment of the body of evidence is based on the GRADE approach. Evidence based on randomized controlled trials began with high-quality evidence, but the confidence in the evidence might be decreased for five reasons, including study design limitations, inconsistency of results, indirectness of evidence, imprecision and publication bias. Evidence based on observational studies start with a “low quality” rating, grading upwards may be warranted for three factors (large magnitude effect, dose-response gradient, or plausible confounding which could reduce a demonstrated effect). The quality of evidence across different outcomes as that associated with the critical outcome with the lowest quality evidence. If the evidence was originated from systematic reviews/meta-analyses, the AMSTAR (A MeaSurement Tool to assess Systematic Reviews) instrument was used for assessment. Only Systematic reviews/meta-analyses fulfilling nine or more of the eleven criteria were regarded as high quality systematic reviews/meta-analyses. The direction and strength of recommendations are determined by all of the evaluation, including the final level for quality of evidence, benefits and harms, patient’s values and preferences and use of resource (13). The guideline panel discussed at modified nominal group meetings for each issue or intervention. The decision was made by votes using the modified Delphi method. The voting method included the following rules (14): in areas of continuing disagreement, a recommendation for or against a particular intervention (compared with a specific alternative) required at least 50% of participants who were in favour of, with less than 20% preferring the comparator (the options could be judged equally). Failure to meet this criterion resulted in no recommendation. For a recommendation to be graded as strong rather than weak, at least 70% of participants were required to endorse it as being strong.
- Declaration of conflict of interest: In the process of compiling the guidelines, panel members participating in the seminars signed a written statement declaring any conflicts of interest with pharmaceutical companies.
- Estimation of favorable and unfavorable factors in the implementation of guidelines: (i) favorable factors: (a) with the popularization and in-depth understanding of evidence-based medicine among Chinese physicians specializing in respiratory diseases, there is an increasing demand for high-quality evidence-based clinical practice guidelines; (b) cough is the most common complaint of patients seeking medical attention; however, many patients are misdiagnosed and do not receive proper treatment. Therefore, their quality of life is badly impaired with an increase in economic burden. This evidence-based guideline for the diagnosis and management of cough satisfies the requirement of clinical practice; (c) the application of the previous two versions of Chinese cough guidelines has established a well-recognized foundation for the utilization of this revision. (ii) Unfavorable factors: (a) due to different understanding of the importance of guidelines and recommendations among physicians of different levels, the promotion, propagation, and implementation of the guidelines in community medical facilities may require continuous efforts; (b) because some diagnostic tests, including the bronchial provocation test, induced-sputum test for differential cell count, and 24-h esophageal pH-multi-channel impedance monitoring, are not available in many hospitals, the potential for application of the guidelines may be limited.
- Time to update: to be revised every 3–5 years.
- Members of guidelines: panel, secretariat, and review groups: see the list at the end of full text in detail.
- This guideline includes an additional Glossary of Terms and List of Abbreviations to facilitate reading (Table 2).
Full table

Full table
Definition, classification, and pathogenesis of cough
Cough is a defensive reflex for clearance of excessive secretions and foreign bodies from airways. However, severe cough frequently affect quality of life badly. Cough is classified into three types based on the duration: acute, subacute, and chronic cough. Acute cough is defined as cough lasting for <3 weeks, subacute cough lasts 3–8 weeks, and chronic cough persists for >8 weeks (6). Cough can also be categorized as dry and wet cough, and a wet cough is defined as sputum volume >10 mL per day. Different types of cough have a spectrum of different underlying causes. Based on chest radiography, chronic cough can be further classified into two subtypes: (I) presence of pulmonary lesions on radiography (for example, pneumonia, tuberculosis, and bronchopulmonary carcinoma), and (II) lack of overt identifiable abnormalities on radiography. This guideline focus on the latter subtype. In China, a majority of patients with chronic cough are 30–40 years old without significant gender preponderance; however, in European and American countries, most patients with chronic cough are 50–60 years old, with a significantly higher incidence in women than in men (15). Chronic cough is related to air pollution (16-19).
The involuntary cough reflex is involved in five sections: the peripheral receptors, vagal afferent nerves, central cough neurons, efferent nerves, and effectors (diaphragm, throat, chest, and abdominal muscles). An effective cough can be induced by stimulation of the irritant receptors of the trachea, bronchopulmonary C fibers, or the mechanically sensitive, acid-sensitive myelinated sensory nerves (Aδfibers). When vagal nerve branches distributed in the upper airway, throat, and esophagus are stimulated, cough can be induced (20). Cough nerve centre is located in the medulla, which is regulated by the cerebral cortex. Cough hypersensitivity is an important pathophysiological mechanism of chronic cough (21-23) related to the activation of transient receptor potentials (TRP), including TRPV1 and TRPA1, and airway inflammation, neural pathways, and nerve center (24-28).
Chronic cough can results in a lot of concomitant disorders, such as incontinence, syncope, insomnia, and anxiety, which involve the cardiovascular, digestive, nervous, urinary, and musculoskeletal systems (2,29).
Medical history and laboratory tests
A thorough medical history and physical examination are important for physicians to develop a differential diagnosis, select laboratory tests, make a tentative diagnosis and empiric therapy (30).
Medical history
Information regarding the duration of cough; phase; characteristics; triggers; effect of altering body position; and concomitant symptoms should be identified. Sputum volume, purulence and characteristics; smoking history; occupational or environmental exposure; medication history, including angiotensin converting enzyme inhibitors (ACEI) or other drugs, can indicate the diagnosis (7) (1D). Occupational cough should be considered when patient has an occupational exposure history. Acute cough is often attributable to the common cold and acute tracheobronchitis, while subacute cough is the result of post-infectious cough (PIC). The timing of cough provides additional diagnostic information. Cough variant asthma (CVA) should be considered for patients with predominantly nocturnal cough. (31,32) (2B). A dry cough indicates a non-infectious cough, while a wet cough is more commonly seen in patients with an infectious cough. Respiratory infectious disease should be considered in patients with a large amount of sputum production or purulent sputum (7,32) (2C). Chronic bronchitis is characterized by mucoid sputum and the cough is usually aggravated in the winter and spring. Tuberculosis, bronchiectasis, and lung cancer should be considered with bloody sputum or hemoptysis. Allergic rhinitis and asthma-related cough should be carefully excluded in patients with a personal or family history of allergy. Upper airway cough syndrome (UACS) should be considered in patients with nasal congestion, runny nose, sneezing, postnasal drip, or post-laryngeal reflux (32) (2C). In the presence of acid regurgitation, belching, or retrosternal burning, gastroesophageal reflux-related cough (GERC) should be considered (32,33) (2C).
Physical examination
Physical examination focuses on the somatotype, nose, larynx, throat, trachea, and lungs; lung sounds; and presence/absence of wheezing, moist rales, and crackles. The possibility of obstructive sleep apnea (OSA)- or GER-related chronic cough should be considered in patients with obesity. A majority of patients with chronic cough have normal findings on a physical examination. Expiratory wheezing suggests the possibility of asthma. Sounds of “velcro opening” at the lower lung lobes may indicate interstitial lung diseases. Inspiratory wheezing may suggest central airway tumor or bronchial tuberculosis. Cardiac signs, including enlargement of heart border, premature beats, and murmurs should also be evaluated.
Relevant additional testing
The main tests include chest imaging, induced-sputum cytology, spirometry, the bronchial provocation test, fractional exhaled nitric oxide (FeNO) measurement, and 24-h esophageal pH-multi-channel impedance monitoring. (I) Imaging: chest radiographs are routinely recommended for chronic cough (2D). The flow chart for the diagnosis of chronic cough should be followed (see supplementary file 1). If an obvious abnormality is observed on plain films, additional investigation is selected based on the characteristics of the lesion. Chest CT can be used to detect lesions anterior and posterior to the mediastinum; small pulmonary nodules; thickening and calcification of trachea; stenosis of the trachea; and enlargement of mediastinal lymph nodes. The uncommon conditions can be identified by radiography, including broncholithiasis, relapsing polychondritis, and bronchial foreign body can be identified by CT (1D). High-resolution CT is helpful for the early diagnosis of interstitial pulmonary diseases and atypical bronchiectasis. If sinusitis is suspected, sinus CT is preferred (34) (2D). Repeated radiographs within a short time span should be avoided. (II) Pulmonary function tests: pulmonary function tests include pulmonary ventilation tests and the bronchial provocation test. These tests are valuable for the etiologic diagnosis of chronic cough and should be routinely used (35-37) (1B). Positive findings on the cough provocation test are important in the diagnosis of CVA. Hospitals unable to perform the cough provocation test can monitor the average peak expiratory flow (PEF) variation overtime (38,39) (1B). An average daily PEF variation of >10% suggests CVA. (III) Induced sputum test: induced sputum test is a safe, well-tolerated, non-invasive method for the etiologic diagnosis of chronic cough and airway inflammation (40-43) (1C). Eosinophilia identified by induced sputum is suggestive of eosinophilic bronchitis (EB), and can also be seen in patients with CVA (40) (1C). Induced sputum cytology can be used to monitor response to inhaled corticosteroids (ICS) in patients with chronic cough (41-43) (1C). The use of 3% hypertonic saline via ultrasonic nebulizer is recommended, but repeated induced sputum tests within 48 hours should be avoided (44-46) (1C) (for details please refer to supplementary file 2). (IV) FeNO measurement: this is a novel non-invasive technology for the diagnosis of airway inflammation. An increase in FeNO (>32 ppb) suggests eosinophilic inflammation or corticoid-sensitive cough (47-54). However, the sensitivity is not high when FeNO measurement is used for screening of eosinophilic inflammation. Approximately 40% of patients with increased numbers of eosinophils have normal FeNO (52-56) (2C). (V) Allergy skin prick tests and serum IgE test: these tests can identify patients predisposed to allergen sensitization and identify specific allergens. They may be useful in the diagnosis of atopic diseases (e.g., allergic rhinitis and atopic cough). Approximately 60–70% of CVA patients and 30% of EB patients are predisposed to allergen sensitization (31,57). (VI) The 24-h esophageal pH-multi-channel impedance monitoring: this is the most commonly useful method of diagnosing gastroesophageal reflux. Dynamic monitoring measures changes of esophageal pH, the number of times the esophageal pH is <4, the longest duration of reflux, and the percentage of time for which the esophageal pH is <4. The grade of reflux is represented as the DeMeester scores. Cough should be recorded in a real-time manner during the monitoring, so that the symptom-associated probability (SAP) between reflux and cough can be calculated (see supplementary file 3 for methods). The non-acid reflux, such as weak acid or weak alkaline reflux, can be detected by esophageal impedance monitoring (2C). (VII) Bronchoscopy: bronchoscopy is not routinely recommended for chronic cough except for the diagnosis is not confirmed by routine tests or in patients with a poor response to the treatment for common causes of cough. Bronchoscopy can be used for the diagnosis or exclusion of uncommon airway conditions associated with cough, including lung cancer, foreign body, tuberculosis, and relapsing polychondritis (10,58-61) (2C). (VIII) Other examinations: peripheral eosinophilia is indicative of atopic diseases, but in most patients with CVA and EB, the peripheral eosinophil counts are within the normal ranges. Severe peripheral eosinophilia (eosinophil count >20%) indicates the possibility of parasitic infections or eosinophilic pneumonia.
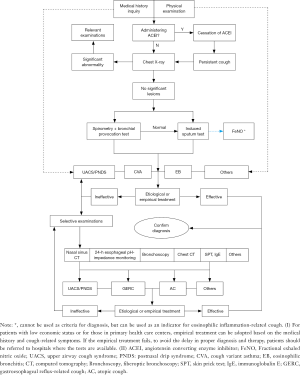
Diagnostic principles and algorithm of cough
Refer to section VI and section VII for the diagnostic flow chart of acute and subacute cough.
The etiological diagnosis of chronic cough should follow the principles as described below (5) (1D): (I) Attention should be paid to the medical history, including ear, nose, and throat, and digestive tract diseases, occupational and environmental exposure, smoking and medication history. The diagnosis can be determined if clinical signs are alleviated after being away from occupational or environmental exposure. (II) Selecting investigations, from simple to complex, based on the medical history. The most common causes of chronic cough are EB and CVA. Together they are responsible for approximately 50% of chronic cough cases (62). Spirometry, the bronchial provocation test, and induced-sputum cytology are recommended as the initial tests for chronic cough (7,40,63) (2B). The measurement of FeNO is recommended as to supplement of the induced sputum test (47-53) (2C). The 24-h esophageal pH-multi-channel impedance monitoring is an important method for the diagnosis of GERC, but it is recommended as the second-line test because it is time-consuming and costly (2D). (III) The common causes of cough, including UACS, CVA, EB, GERC, and atopic cough (AC) should be initially considered as the most possible etiology for chronic cough (37,62,64-69). Bronchoscopy is valuable in the diagnosis of uncommon causes of chronic cough. (IV) Diagnosis and management can be implemented simultaneously or sequentially. If certain tests are unavailable, the treatment should be based on the clinical characteristics and the therapeutic response (30). Further evaluation should be considered if patients fail to respond to the treatment (2C). With typical symptoms of rhinitis, sinusitis, or postnasal drip, treatment for UACS should be initially prescribed. If patients present with symptoms related to gastroesophageal reflux cough after eating food, treatment for GERC should be given empirically. (V) Response to the treatment is the prerequisite for confirming etiologic diagnosis. When the cough is partially relieved, the factors affecting the effectiveness of treatment or other causes of chronic cough, such as UACS concurrent with GERC, CVA, or EB, GERC concurrent with EB or CVA should be evaluated (2C). (VI) When the treatment is ineffective, the following factors should be evaluated: diagnosis, therapeutics, and occupational or environmental exposure (2C).
Flow chart for the etiologic diagnosis of chronic cough (see Supplementary file 1)
Assessment of cough
The assessment of cough includes: the visual analogue scale (VAS), cough symptoms score, quality of life questionnaire, cough frequency monitoring, and the cough provocation test. These tests are used to monitor the disease status and treatment efficacy (29,70).
The VAS scoring system
Patients mark a point on a straight line corresponding to their perception of the severity of cough. The score ranges from 0–10 cm (0–100 mm), with 0 representing minimal severity and 10 representing extreme severity. Compared with the cough symptoms score, the intervals between grades with the VAS are smaller, which is helpful for longitudinal comparison before and after treatment (29,70,71).
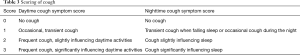
Coughing score
This is a quantitative scoring system of cough used to assess the severity of cough and efficacy of treatment. Daytime and nighttime scoring is done, however it may be difficult to discriminate between grades (29,70,71) (see Table 3 for details).
Full table
Quality of life questionnaire
The Chronic Cough Impact Questionnaire (CCIQ), Cough-Specific Quality of Life Questionnaire (CQLQ), and Leicester Cough Questionnaire (LCQ) are specific for chronic cough and demonstrate good reliability, validity, and responsiveness. These questionaires are important in the assessment of cough severity and efficacy of treatment (29,71-81). The Chinese version of the LCQ is recommended to assess cough-related quality of life (77) (1A).
Coughing frequency monitoring
The cough symptoms score, VAS, and quality of life questionnaire are subjective assessment tools. Cough frequency monitoring is used for evaluation of cough severity and treatment efficacy (82-84). There is diversity in tolerance of patients to coughing, and cough frequency does not definitively correlate with cough severity. Since cough frequency monitors are not available in China, their clinical applications are limited.
Cough provocation test
This test is used to assess therapeutic efficacy and study cough mechanisms. It is not a routine test in clinical practice. Patients inhale nebulized aerosol particles, which stimulate the cough receptors. Capsaicin is commonly used for the cough provocation test (see supplementary file 4 for test methods). The cough sensitivity is expressed as the cough threshold C5, defined as the lowest concentration of capsaicin inducing ≥5 coughs (C5).The concentration that induces ≥2 (C2) or ≥5 (C5) coughs is an indicator of cough sensitivity. In China, the reference value for C5 in the capsaicin provocation test in normal subjects is ≥125 mol/L (22). Increased cough sensitivity is an important characteristic of chronic cough, and is frequently observed in AC, GERC, UACS, CVA etc. (23). In addition, significantly increased cough sensitivity is identified in viral post-infectious cough (VPIC) (21,85). The cough provocation test is safe, well tolerated, and repeatable, and is useful in identifying patients with cough hypersensitivity, and to quantitatively evaluate chronic cough. However, the cough provocation test cannot be used to assess cough frequency and severity (21,85-89). In Europe and USA, women have higher cough sensitivity than men (90,91) .
Diagnosis and management of acute cough
A thorough evaluation of medical history, physical examination, and specific diagnostic tests can be used to exclude severe diseases as a cause of acute cough. Acute cough may be an early sign of serious disease, including acute myocardial infarction, left heart failure, pneumonia, pneumothorax, pulmonary embolism, and foreign body aspiration (92-97). The common cold, acute tracheobronchitis are the common causes of acute cough. Exacerbation of asthma, chronic bronchitis, or bronchiectasis may lead to acute cough. An increasing proportion of acute cough is related to occupational or environmental exposures.
Common cold
A viral infection is the cause of the common cold (96,98,99). The diagnosis is based on history and physical examination, viral culture, and/or serological testing; chest imaging is not necessary (96,100) (1D). In addition to cough, nasal discharge, sneezing, nasal congestion, postnasal drip, throat irritation and clearing the throat are common symptoms associated with the common cold. Systemic symptoms are not common (94,98-103); this is in contrast to the flu that is associated with fever and myalgia as well as cough (101-104).
Symptomatic treatment is used for the common cold. (I) Antibiotics are infective in decreasing the duration or relieving symptoms. Antibiotics may lead to concurrent adverse effects. Antibiotics are not recommended for patients with the common cold (96,105-109) (1A). (II) Decongestants: decongestants in adults can rapidly relieve nasal congestion with minimal adverse side effects. (III) Antihistamines: the use of first-generation of antihistamines as a single therapeutic agent is not recommended due to insufficient of clinical benefits (110-113) (1A). Combined treatment with decongestants and first-generation antihistamines (brompheniramine maleate and pseudoephedrine) can significantly improve cough, sneezing, and nasal discharge in adult and adolescent patients (114-116) (2A). However, adverse reactions should be monitored. Pseudoephedrine in pediatric patients should be used with caution (110,112,113). (IV) Antipyretics and analgesics: these medicines are prescribed for symptomatic relief of fever, sore throat, and muscular aches in patients with the common cold (1A). Acetaminophen is the most widely used antipyretic and analgesic agent in clinical practice. Non-steroid anti-inflammation drugs (NSAIDs) are not recommended for patients without fever, headache, or muscular ache (100,117-123) (1A). (V) Antitussive: for patients with severe cough, central or peripheral acting antitussive agents can be used. Central-acting agents (i.e., dextromethorphan, codeine) have limited efficacy to suppress cough in patients with the common cold and should not be used alone (100,111,113,124) (2D). A combination of first-generation antihistamines and an antitussive agent is recommended for cough due to the common cold (100,113,125-130) (1A). (VI) Ipratropium bromide: nasal spray can improve runny nose and sneezing in adult and adolescent patients, however, adverse events including nasal dryness, nasal congestion, and epistaxis may occur (131,132) (2A). Although TCM treatment may be useful in treating the common cold, high-quality clinical evidence are lacking (133,134).
Acute tracheobronchitis
Acute tracheobronchitis is characterized by an inflammation of tracheobronchial mucosa due to biological or non-biological factors. The most common etiology is a viral infection, including rhinovirus and influenza virus; bacterial etiology is less common (99,135-141). Cold air, dust, and irritant gases can cause acute tracheobronchitis. Most cases are self-limited, however, infants and the elderly patients may develop refractory bronchitis.
Clinical manifestations
Symptoms related to upper respiratory tract infection, such as fever, headache, muscle aches and pains, sore throat, rhinorrhoea, sneezing are noted initially. Cough become progressively severe, with or without sputum production. Purulent sputum indicates bacterial infection. Systemic symptoms can resolve in several days, while cough and sputum production may last for 2–3 weeks. No obvious abnormalities or only slight increase of lung markings can be observed in chest radiographs. Coarse breath sounds, with wet or dry rales, can be heard in both lung fields.
Diagnosis and differential diagnosis
The diagnosis is established by clinical manifestations. Viral culture, serology, and sputum tests are not required (136,142-144) (1D). Acute tracheobronchitis should be considered for patients with cough within 3 weeks and with or without sputum production, when the common cold, pneumonia, asthma, and acute exacerbation of chronic obstructive pulmonary disease (COPD) have been excluded (96,142,144-149) (1D). In patients with a tentative diagnosis of acute tracheobronchitis, the likelihood of developing pneumonia is low if the heart rate is ≤100 beats/min, respiratory rate ≤24 times/min, body temperature of ≤38 °C and the chest radiograph is normal (96,147,150-152) (3C).
Treatment
Symptom-targeted therapy is the main principle of treatment. Adequate use of antitussive agents should be considered for patients with severe dry cough, and expectorants or mucolytic agents can relive cough in patients with difficulty expectorating sputum (153-158) (1B). Sustained-release guaifenesin can improve symptoms related acute respiratory infection (153,155,156) (2A). Data from lots of studies showed that it is not necessary to use antibiotics regularly due to the unclear efficacy (96,105,107,147,159-167) (1A). For patients with purulent sputum, antibiotics are recommended (1D). For patients with acute bronchitis, if antibiotics are not prescribed, the rationale should be explained since patients may request antibiotic treatment due to their previous experience and expectations (168-174) (1B). When there is evidence of a bacterial infection, such as purulent sputum or increased level of peripheral leukocyte count, antibacterial agents are selected based on the presumed pathogens and the results of antibiotic sensitivity tests. Prior to obtaining the culture and sensitivity results, oral antibiotics such as β-lactams and quinolones can be used (7). Regular use of β2-agonist is not necessary, however, for adult patients with acute bronchitis and concomitant asthma, β2-agonist may be beneficial (175) (2A). Currently no high-quality data are available to verify the safety and efficacy of TCM in the treatment of acute bronchitis (176,177).
Diagnosis and management of subacute cough
The most common cause of subacute cough is PIC, followed by CVA, EB, and UACS (178,179) (1B). For management of subacute cough, the first step is to determine if the cough is secondary to a previous respiratory infection and if empirical treatment is required. If the treatment is ineffective, other causes should be considered using the diagnostic process for chronic cough. A diagnosis of PIC is made on the basis of a history of the common cold, upper respiratory tract infection, and protracted cough (178). The bronchial provocation test and induced sputum test are recommended if available (2C). A few cases of “refractory PIC” may be EB, CVA, and GERC (178).
If the cough persists for 3–8 weeks after the acute symptoms of respiratory infection resolve, and the patient has an irritating dry cough or a cough with a small amount of mucoid sputum, and normal chest radiograph (103,179-181), then PIC should be considered. Viral infection is the most common trigger of PIC. Patients with a history of PIC and cough hypersensitivity are more predisposed to PIC (178,180).
PIC is usually a self-limiting disease. However, a few patients present with refractory cough and may progress to chronic cough. Post-viral infection cough may not require antibacterial treatments. For the patients with severe cough, antitussives, antihistamines, and decongestant are recommended for short-term use (2C). Methoxyphenamine is effective for PIC (182) (2C). Since montelukast is not recommended for PIC (183) (2B). The efficacy of ICS for the treatment of PIC is not certain, therefore not recommended (184,185) (2B). In TCM it is believed that PIC is caused by “the evil wind invading the lung, causing an obstruction of qi,” therefore the treatment should follow the principles of “dispelling the wind and opening the inhibited lung-energy,” and “antitussive and relieving the sore throat.” The Suhuang Zhike capsules, comprised of Chinese ephedra, purple perilla leaf, lumbricus, folium eriobotryae, and perilla fruit is an effective treatment for PIC (186) (2C).
Protracted cough is commonly caused by Mycoplasma pneumoniae or Chlamydia pneumoniae. Haemophilus influenzae and Streptococcus pneumoniae are more common pathogens in infants, the elderly, and susceptible patients (187-189). Serological antibody test is the most effective method for diagnosing mycoplasma or chlamydia infection. Serology is helpful for early diagnosis and is routinely used in clinical settings (190,191) (1C). Serum cold agglutinin titers of ≥1:64 or mycoplasma IgM antibody titer with four-fold increase from the acute to the recovery phase indicates a recent infection with M pneumoniae (190) (2D). A serum chlamydia antibody titer elevation with four-fold increase from the acute to the recovery phase or the antibody titer of IgM ≥1:16 or IgG ≥1:512 at any single time point are helpful to verify chlamydia infection. Macrolides or quinolones are effective for protracted cough caused by the M pneumoniae and infection pneumonia (7) (2C). Amoxicillin or cephalosporin can be used for 2–3 weeks to treat protracted cough due to infection with Gram-positive cocci (192,193) (2B).
For adolescent and adult patients, pertussis (“whooping cough”) should be considered when the Bordetella pertussis antibody titer is increased (194-196) (2C). Typical symptoms of pertussis, such as paroxysmal cough, vomiting after coughing, and inspiratory wheezing, are of limited value in the clinical diagnosis of pertussis (197,198) (2A). Anti-pertussis immunoglobulin G (anti-PT-IgG), polymerase chain reaction (PCR), and bacterial culture are helpful in the diagnosis of pertussis (199-204) (2C).
Once pertussis is diagnosed, early treatment with macrolides should be initiated. With treatment during the catarrhal phase, 1–2 weeks before coughing paroxysms occur, symptoms may be lessened. Although treatment cannot affect the natural progress of pertussis, it can reduce the severity of the disease (11,205) (1B). Antibiotics are not recommended for patients with pertussis in the non-catarrhal phase (protracted phase) (206) (1A). Corticosteroids, β2-adrenergic receptor agonists, pertussis specific immunoglobulins, and antihistamines are not recommended (207) (1A).
Diagnosis and management of chronic cough due to common etiology
Common causes of chronic cough including CVA, UACS, EB and GERC should be initially considered when diagnosing chronic cough (1A); AC is also a common cause of chronic cough. The common causes account for 70–95% of cases of chronic cough (62,64,66-69). Since a majority of patients with chronic cough are not related to infection (208), antibiotics should be not be used (1C).
Upper airway cough syndrome (UACS)—postnasal drip syndrome (PNDS)
PNDS is characterized by cough, which may result from the direct or indirect stimulation on the cough receptors in the postnasal and pharyngeal areas. Because it is not clear that upper airway-related cough is caused by direct stimulation of postnasal dripping or stimulation on upper airway cough receptors by inflammation, the ACCP Guidelines for Diagnosis and Management of Cough [2006] suggested using the term upper airway cough syndrome (UACS) to replace PNDS (209). However, this change in terminology remains controversial (210). Since PNDS is more intuitive and visual in a certain proportion of patients with typical postnasal dripping, this guideline continues to use PNDS.
UACS/PNDS is one the most common causes of chronic cough, generally related to rhinitis and sinusitis. The diagnosis of UACS/PNDS are confirmed by the effectiveness of empirical treatment (62,211-213) (1B). In addition to nasal diseases, UACS/PNDS may be related to throat and pharynx diseases, including chronic laryngopharyngitis and chronic tonsillitis (7,211). Chronic cough may also be caused by cough hypersensitivity (214,215).
Clinical manifestation
(I) Symptoms: in addition to cough and sputum production, other symptoms include nasal congestion, excessive nasal secretions, frequent throat clearing, post-laryngeal mucus adherence, and postnasal dripping. Allergic rhinitis can also present with nasal itching, sneezing, and watery nasal mucus production. Rhino-sinusitis usually presents with nasal congestion, purulent nasal mucus, and can be accompanied occasionally with facial ache/swelling, and abnormal sense of smell (216). (II) Signs: the common signs of allergic rhinitis include pale or swollen nasal mucosa, and clear or sticky mucus in the nasal tract and in the bottom of nasal cavity. For non-allergic rhinitis, the nasal mucosa shows hypertrophic or congestive changes, and the oropharyngeal mucosa may have “cobblestone-like changes” or post-pharyngeal mucus adherence. (III) Auxiliary examinations: sinus imaging may reveal signs of chronic sinusitis, including mucosal hypertrophy and fluid within the sinus cavity. Seasonal cough suggests the possibility of contact with specific allergen (such as flower pollen, dust mites) and the allergen skin prick test is helpful for the diagnosis. Chronic sinusitis can be divided into subtypes based on etiology: viral, bacterial, fungal, and allergic sinusitis. Nasal polyps may occur with UACS/PNDS. If sinusitis is suspected, CT imaging should be initially performed, and nasal endoscopy, allergen skin prick tests, and immunological tests should be selected when necessary.
Diagnosis
The etiological causes of UACS/PNDS can involve several conditions of the nose, sinus, pharynx, and throat and multiple nonspecific symptoms and signs. Diagnosis should be made based on the history, physical examination, and tests. Effective treatment to the underlying disease, following by exclusion of lower airway diseases or GERC is recommended. The criteria for diagnosing UACS/PNDS are (6) (2C): (I) paroxysmal or persistent cough, often in the daytime and rare after sleep; (II) history and clinical manifestations of nasal and/or throat conditions; (III) auxiliary tests supporting nasal and/or throat conditions; (IV) cough improve after specific therapy. Treatment should be determined based on potential conditions related to UACS/PNDS.
Treatment
(I) Etiological therapy: (i) for non-allergic rhinitis and the common cold, first-line treatment consists of the first-generation antihistamines and decongestants (1A), which are efficacious in most patients within several days to two weeks; (ii) intranasal ICS, including budesonide, fluticasone propionate and betamethasone acetate, and oral second-generation antihistamines is used for allergic rhinitis (217) (1A). Second-generation antihistamines include loratadine, desloratadine, and desloratadine citrate disodium. If second-generation antihistamines are not available, first-generation antihistamines can be used with the similar clinical response except for the greater drowsiness. Leukotriene receptor antagonists are effective to allergic rhinitis (218,219) (1A). For patients with severe allergic rhinitis that fails to respond to routine treatment, immunological therapy to specific allergens may be effective. However, immunological therapy require a longer time to demonstrate effectiveness (217,220,221) (2B). (iii) Chronic sinusitis: (a) bacterial culture of nasal secretions in patients with chronic sinusitis primarily identify Staphylococcus aureus, Staphylococcus epidermis, and Streptococcus pneumonia that are generally colonized bacteria related to acute onset of disease. Moreover, culturing bacterial colonies can result in the formation of bacterial biofilms (222,223). In general, bacterial sinusitis is caused by a mixed infection, and broad-spectrum antibiotic therapy is necessary. The antibacterial spectrum should cover Gram-positive, Gram-negative, and anaerobic bacteria. For patients with acute onset, the therapeutic duration should be no less than 2 weeks, and for patients with chronic diseases treatment should be longer than 2 weeks. Amoxicillin/clavulanic acid, cephalosporins, or quinolones are the most commonly used antibiotics (5). (b) Evidence to support the efficacy of long-term low-dose macrolides for chronic sinusitis treatment is limited (224,225). Long-term treatment with macrolides are not recommended (3B). (c) Combined regimens with nasal ICS for more than 3 months are recommended. For patients with chronic sinusitis and coexisting nasal polyps, ICS can be used to avoid unnecessary operation (226) (1A). Sequential treatment with ICS following oral steroids has better efficacy than ICS alone (227) (2A). d. It is uncertain whether surgical or medical treatment has a better response (228). However, when medical treatment is not satisfactory, nasal endoscopic surgery can be considered (229) (2B). (II) Symptom-targeted treatment: (i) local decongestants can relieve the congestion and swelling of the nasal mucosa and help drainage of secretions, and alleviating the nasal congestion. However, long-term use is not recommended because of the potential to develop medicamentous rhinitis. The therapeutic course of nasal decongestant spray is less than 1 week (230-233) (1B). The combination of first-generation oral antihistamines plus decongestants is recommended for 2–3 weeks (234-238) (2D). (ii) Mucolytics (carbocisteine/erdosteine) may be beneficial for chronic sinusitis (239-241) (2B). (iii) Nasal washing with normal saline can provide an effective therapy for chronic nasosinusitis and chronic rhinitis (242-246). Avoidance or reduction of the exposure to allergens is helpful to relieve the symptoms of allergic rhinitis.
CVA
CVA is an atypical form of asthma and one of the most common causes of chronic cough (37,64,66-69). It presents with cough as the predominant or sole symptom. There is no wheezing or dyspnea, expect to bronchial hyperresponsiveness. A multicenter prospective study showed that CVA accounted for 33% of chronic cough in China (62). In some patients, cough may be the sole or predominant symptom despite significant impairment in lung function. Cough may become the predominant symptom after wheezing has improved in classic asthmatics (247).
Clinical manifestation
Patients have severe, irritating dry cough, particularly at night or early morning (32). The patients are sensitive to cold air, dust, odors, and smoke, but these factors alone can trigger other causes of chronic cough (32).
Diagnosis
Diagnosis is based on history, physical examination, the bronchial provocation test, and the response to asthmatic treatment. Responding well to bronchodilators is an important feature of CVA. However, a few CVA patients (approximately 30%) are not responsive to bronchodilators alone (248,249) and the response to bronchodilators is not necessary as a diagnostic criterion of CVA (2B). Average PEF variation can be selected for diagnosis, if the bronchial challenge test is not available. Sputum eosinophilia and elevated FeNO level suggest a diagnosis of CVA (40,47,50,53).
The following diagnostic criteria for CVA are recommended (1A):
- Chronic cough, usually with irritating cough occurring during the night;
- Positive bronchial challenge test (fall in FEV1 from baseline of ≥20% with 12.8 micromole of methacholine or with 7.8 micromole of histamine), or average daily diurnal PEF variability ≥10% over 2 weeks, or positive bronchodilator reversibillity test (increase in FEV1 ≥12% and 200 mL from baseline, 10–15 minutes after 200–400 mcg albuterol or equivalent);
- Cough resolved after asthma treatment.
Treatment
The therapeutic principles for CVA are the same as those for typical asthma. (I) Combined treatment with ICS plus bronchodilators can improve the cough more rapidly and effectively than treatment with ICS or a bronchodilator alone (250,251). A combination of ICS and bronchodilator (β2 receptor agonist), such as budesonide/formoterol and fluticasone/formoterol, is recommended (1B). The treatment should last for more than 8 weeks, and in some patients, long-term treatment may be required (2D). (II) A short-term oral corticosteroid (10–20 mg/d, for 3–5 days) is recommended for patients refractory or less responsive to ICS treatment, or in patients suffering from severe inflammation of the airway (2C). If patients are not responsive to oral corticosteroids, the patient should be evaluated again. A false positive provocation test or the existence of other diseases, such as early-stage eosinophilic granulomatosis with polyangiitis and GERC, should be considered. (III) Leukotriene receptor antagonists are effective in improving cough, airway inflammation, and the quality of life (252-256) (2B). For a minority of patients refractory to treatment with ICS, leukotriene receptor antagonists may be effective. However, the treatment course and effects of inhibition on airway inflammation need to be further studied. (IV) In TCM, CVA is caused by “the evil wind invading the lungs”, so the treatment should follow the principles of traditional medicine in “dispelling the wind and opening the inhibited lung-energy” and “antitussive and reliving the sore throat.” The Suhuang Zhike capsule is effective for treating CVA (257) (2B).
Prognosis
About 30–40% patients with CVA will develop typical asthma. A longer duration of disease, higher airway hyperresponsiveness, and higher level of eosinophils in induced sputum are the risk factors for developing classic asthma. Long-term use of ICS may be helpful to prevent development into typical asthma (258-260) (2B).
EB
EB is one of common causes of chronic cough and accounts for 13–22% of chronic cough (37,65,66,69). Characteristics of EB include chronic eosinophilic inflammation of airway, inflammation of the central airway, and with more infiltration of mast cells which is located more in the airway smooth muscle cells. These pathologic features may explain why there is a lack of bronchial responsiveness in EB. The degree of inflammation and level of oxidative stress are lower than in patients with CVA (261-264). Approximately one-third of the patients have concurrent with allergic rhinitis (57,65).
Clinical manifestations
Patients present with dry cough or a little mucoid sputum that is more common during the day. Patients are often sensitive to smoke, dust, odors, and cold air, which induce cough. Patients do not have wheezing or dyspnea. The pulmonary ventilation function and variations of PEF are normal without signs of airway hyperresponsiveness.
Diagnosis
EB shares similar clinical features to CVA. Sputum eosinophilia is the key to diagnosis, with eosinophils being more than 2.5% of cells in sputum (63). The sensitivity of FeNO is not high for diagnosis of EB, but FeNO of >32 ppb suggests eosinophil-related chronic cough (e.g., EB or CVA) (48,50,52,54) (2C). Exposure to ocyanic acid and chloramine in flour can induce EB (265-270) (2C). Occupational factors, the history, eosinophil counts in induced sputum (or bronchoalveolar lavage fluid), airway responsiveness, and the response to steroid treatment should be considered when diagnosing EB, (1B). The following diagnostic criteria are recommended: (I) chronic cough, presenting as irritating dry cough or with bit amounts of sticky sputum; (II) unremarkable chest radiographic findings; (III) normal pulmonary ventilation function, a lack of airway hyperresponsiveness, and normal average weekly PEF variation; (IV) sputum eosinophil count ≥2.5%; (V) exclusion of other diseases with eosinophilia; (VI) cough improves after treatment with corticosteroids.
Treatment
EB patients respond well to corticosteroids. Cough will be resolved or relieved shortly after the treatment. The use of ICS is the first therapeutic option, and treatment course with more than 8 weeks is recommended (2C). Initial treatment is a short course of oral prednisone (10–20 mg/d for 3–5 days) (7) followed by ICS. If the patients show no response to the treatment with low-dose corticosteroids, systemic diseases related to eosinophilia, including hypereosinophilic syndrome and eosinophilic granulomatosis with polyangiitis, should be considered.
Prognosis
Over half of the EB patients will relapse after treatment. Patients with concurrent rhinitis and persistent eosinophilic inflammation are at risk for recurrence (57). Previous reports showed that a small proportion of patients with EB develop chronic airway obstructive diseases (asthma or COPD) (271-273). A recent study with a large sample and long-term follow-up demonstrated that only 5.7% patients with EB would develop asthma, suggesting that EB might be a distinct disease and not an early stage of asthma, COPD (57).
GERC
GERC is a special kind of gastroesophageal reflux disease that presents with chronic cough as the sole or predominant symptom. Studies have showed that GERC is a common cause of chronic cough (35,62,64-67). The prevalence of patients with GERC is lower in China than that in Western countries. The pathogenesis of GERC involves microaspiration, esophageal-bronchial reflux, esophageal motility dysfunction, autonomic nervous system dysfunction, or neurogenic airway inflammation (274-277). Esophageal-bronchial reflux plays an important role in GERC. In addition to gastric acid reflux, cough in some patients may be related to abnormal nonacid reflux that is a weakly acidic or alkaline reflux, such as bile reflux.
Clinical manifestations
Apart from cough, 25–68% of patients with GERC have classical reflux symptoms, such as regurgitation, heartburn, and belching. However, cough may be the sole symptom in some patients (33,278). Cough generally occurs after meal, daily in an upright position. The cough is usually nonproductive (dry) or accompanied by small amounts of mucoid sputum, and is triggered or aggravated by ingestion of acidic or fatty foods (33,279).
Diagnosis (2C)
(I) Chronic cough primarily during the day. (II) 24-h ambulatory esophageal pH monitoring or multi-channel intraluminal impedance-pH monitoring (MII-pH) shows a DeMeester score of ≥12.70 (280) and symptom association probability (SAP) of ≥80% (281). A symptom index of ≥45% is useful for the diagnosis of GERC (282). (III) Cough resolves or disappears after anti-reflux treatment.
It should be noted that negative findings of 24-h ambulatory esophageal pH monitoring do not exclude GERC as cause of cough. In a minority of patients with concurrent or predominant nonacid reflux (e.g., bile reflux) or intermittent reflux, the results of the ambulatory esophageal pH monitoring may be normal. Esophageal pH monitoring combined with intraluminal impedance can identify nonacid reflux (2C). When 24-h ambulatory esophageal pH monitoring or MII-pH is not available, the followings indicate GERC: (I) cough related to eating, such as coughing after or during meal; (II) typical reflux symptoms, including heartburn, regurgitation, or a score of Gastroesophageal Reflux Disease Questionnaire (GerdQ) of ≥8; (III) no evidence of CVA, UACS, or EB, and the cough does not improve with treatment for CVA, EB, or UACS. In patients who meet the above criteria, GERC should be considered as a cause of chronic cough, and diagnostic/empirical therapy for GERC may be initiated (7,32,283) (2B).
Proton pump inhibitor (PPI) is recommended for patients suspected to be due to GERC (284) (2C). A standard or intensive dose of PPI (e.g., omeprazole 20–40 mg, twice daily) should be prescribed for no less than 2 weeks. If cough disappears or is significantly improve after reflux treatment, GERC can be determined. However, GERC cannot be ruled out if patients fail to improve with PPI treatment. When compared with the laboratory investigations (24-h ambulatory esophageal pH monitoring or MII-pH), a trial of PPI is simpler and more cost-effective (284), but has the disadvantage of lower specificity.
Treatment
(I) Lifestyle modification: weight loss is recommended for the overweight patients. Patients should avoid late-night meals, and foods which are acidic, spicy, or fatty, coffee and acidic beverages, smoking and strenuous exercise (2D). (II) Antacids: acid suppression is recommended as the standard treatment for GERC (285-287) (1A). Common choices are either PPI (omeprazole, lansoprazole, rabeprazole and esomeprazole, etc.) or H2 receptor antagonists (ranitidine or other equivalent drugs). Generally, PPI are superior to H2 receptor antagonists in acid suppression and symptom relief, and should be administered 30–60 min pre-prandially (288), with a treatment course of at least 8 weeks. (III) Prokinetic agents: most patients with GERC have esophageal motility dysfunction, therefore the addition of prokinetic agents (domperidone and mosapride) is recommended (289) (1D).
If standard antireflux therapy fails to resolve the chronic cough in patients with evidence of reflux, the dosing scheme and treatment course should be reviewed. In addition, refractory GERC due to nonacid reflux should be considered. The persistent cough may not be related to reflux or may be caused by multiple etiologies (290-293). If the treatment fails, it is recommended to perform 24-h ambulatory esophageal pH monitoring or MII-pH again to exclude the possibility of under-treatment or misdiagnosis (2C).
Refractory GERC can be treated with baclofen. Adverse effects of baclofen include drowsiness and fatigue (291,294-296) (2C). When the treatment with the standard dose of PPI is not effective, increasing the dose of PPI may be helpful (297-300) (2A). If treatment with one kind of PPI fails, switching to another PPI may be effective (301) (2C). Combining H2 receptor antagonist with PPI may ameliorate cough symptoms due to refractory gastroesophageal reflux or nighttime acid reflux (302) (2C). If necessary, a consultation with gastroenterology specialists can determine the optimum therapeutic regime. For a minority of patients with severe reflux resistant to pharmacological treatment, antireflux surgery (primarily laparoscopic fundoplication) or endoscopic therapies may be effective (303-314) (2C). Currently, no data are available that directly compare the efficacy of endoscopic therapy with medical management. Because of postoperative complications and the potential for relapse, surgical indications should be clearly defined. It is recommended that antireflux surgery be considered only when the cough is poorly controlled, severely impacts the patient’s quality of life, and despite acid suppression, significant residual reflux identified by 24-h ambulatory esophageal pH monitoring or MII-pH (2D).
AC
AC is a kind of chronic cough that characterized by atopy, and response to corticosteroids or antihistamines, but no sputum eosinophils and airway responsiveness. Patients present with an irritating, paroxysmal dry cough, that occurs during the day and night. Cough can be induced by smoke, dust, cold air, and talking, and is usually accompanied by itching of the throat.
The surveys conducted in China demonstrated that AC was one of the common causes of chronic cough (37,62,66,69). In patients with chronic cough, without airway hyperresponsiveness, and a sputum eosinophilia, AC should be considered as the possible cause of cough (2C). The pathogenesis of AC has not been fully elucidated.
Japanese researchers reported that anti-fungal treatment is effective in fungus-induced cough where fungi have colonized the airway, serving as allergen (315). It is unclear if fungal-related cough occurs in other countries and regions and further studies are needed.
The following diagnostic criteria are recommended (2C)
(I) Chronic cough, primarily dry, irritating cough. (II) Normal pulmonary ventilatory function and bronchial responsiveness. (III) Lack of sputum eosinophilia. (IV) Presence of one of the followings: (i) a history of allergic diseases or exposure to allergens; (ii) positive allergen skin prick test; (iii) increased level of total serum or positive specific IgE. (V) Clinical response to corticosteroids or antihistamine treatment.
Treatment
Corticosteroids, antihistamines, or a combination of both are the treatment options. The treatment course of ICS should last for more than 4 weeks, and oral corticosteroids can be used initially for a short period (3–5 days) for treatment (7,10) (2C).
Diagnosis and management of chronic cough of the other etiologies
Chronic bronchitis
Chronic bronchitis is defined as a productive cough that lasts for 3 or more months per year for at least two consecutive years, after the other causes of chronic cough have been excluded. The cough is aggravated in the winter, with white foamy or mucoid sputum. Nocturnal cough may also occur during acute exacerbations.
Based on the epidemiological investigation, chronic bronchitis is common, but accounts only for a small proportion of the patients with chronic cough referred to specialty clinics. The discrepancy may be related to the lack of objective standard for the diagnosis of chronic bronchitis, therefore patients with chronic cough due to other diseases may be misdiagnosed as chronic bronchitis. Since chronic bronchitis is an early stage or subtype of COPD, the severity of chronic cough and sputum is associated with an increased frequency of acute exacerbation and increased mortality (316,317).
Investigations in Asian regions demonstrated that acute exacerbations of chronic bronchitis are generally caused by Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Epidemiological studies should be conducted to determine antibiotic resistance in those regions, providing guidance for selection of antibiotics (318-322) (1B). Moxifloxacin and levofloxacin are the commonly used antibiotics for the treatment of acute exacerbation of chronic bronchitis due to their broad anti-bacterial spectrum and few side effects (318,320,321) (2B).
Bronchiectasis
Bronchiectasis is characterized by irreversible bronchial enlargement and distortion due to the chronic inflammation. The primary abnormality is in the subsegments of the bronchi. Typical clinical manifestations include chronic cough, production of mucoid or purulent sputum, and intermittent hemoptysis, which may coexist with chronic rhinosinusitis. Diagnosis for patients with a typical medical history is not challenging, whereas misdiagnosis can easily occur in patients with mild bronchiectasis and unclear medical history. In patients with suspected bronchiectasis, positive findings in the chest radiographs (such as curly hair sign) may suggest the diagnosis, but high-resolution chest CT is the best method to confirm the diagnosis (323-325) (1C).
Regular use of ICS is not recommended for the patients with stable bronchiectasis (325-332). Nonetheless, the combination of ICS with long acting beta agonists (LABA) or long acting antimuscarinics (LAMA) may improve cough in patients with bronchiectasis who have chronic airflow obstruction or airway hyperresponsiveness (323,324,333) (1A). Postural drainage is helpful in eliminating the accumulated sputum (2D). Intravenous administration of antibiotics is recommended in patients with severe conditions, including highly pathogenic bacteria resistant to oral antibiotics, or failure to respond to oral antibiotics (331,334,335) (2B). Macrolides are helpful to improve symptoms and reduce the risk of acute exacerbation in patients with stable bronchiectasis, bacterial resistance and the adverse effects of long-term antibiotics administration should be considered (336-340) (2B). Regular inhaled mucolytics are not recommended (341) (1A). Statins (342,343) (2B) and mannitol inhalation may also help manage bronchiectasis, but this is not routinely recommended (344-347) (2B).
Bronchial tuberculosis
Bronchial tuberculosis is not rare among patients with chronic cough, in China. Bronchial tuberculosis is generally co-existed with pulmonary tuberculosis, but may exist alone in a considerable proportion of patients. The main symptoms are chronic cough, possibly concomitant with tuberculosis-associated symptoms including low-grade fever, night sweats, and emaciation. For some patients, cough is the only manifestation. Localized inspiratory, dry rales can occasionally be heard. In clinical practice, patients with bronchial tuberculosis and normal chest radiographic findings may be misdiagnosed and under diagnosed (348-352).
If bronchial tuberculosis is suspected, sputum smears hould be conducted first for the detection of acid-fast bacillus. Patients may have a Mycobacterium tuberculosis-positive sputum culture. Pulmonary radiographic signs may be minimal, whereas abnormal findings of the trachea and main stem bronchi, including bronchial wall thickening, airway stenosis, and obstruction can be detected. Bronchial lesions especially lesions located in the subsegmental bronchi may be identified by CT. Computed tomography, particularly high-resolution CT, is more sensitive than radiographs. Bronchoscopy is the important approach to confirm bronchial tuberculosis, which has a high positive rate when combined with routine brushing and tissue biopsy (353) (2C).
Cough due to ACEI or other medications
Cough is a common adverse event of ACEI, the incidence is 5–25% in patients taking the drugs, and ACEI-induced cough accounts for 1.7–12% of chronic cough (354-359). The independent risk factors are smoking, previous ACEI-induced cough (360), and nationality (East Asian or Chinese population) (361). Cough is not related to the age or sex of the patient (362) or the dose of ACEI (363).
The diagnosis can be confirmed when cough resolves after ACEI cessation. Usually, cough will disappear or be significantly relieved within 1–4 weeks after discontinuing the drug (364) . For patients with prior administration and the current risk of ACEI-induced cough, angiotensin II receptor antagonists can be used to replace ACEI for the treatment of the underlying disease (2D).
Cough can also be caused by mycophenolate mofetil, macrodantin, propofol, β-receptor blockers, leflunomide, simvastatin, γ-interferon, and omeprazole (29,365-367).
Bronchogenic carcinoma
Cough is an early-stage and common symptom of central bronchogenic carcinoma, with an incidence of 25–86% (368-370). Chest radiographs may be normal in the early stage, therefore misdiagnosis and under diagnosis may occur. In patients with a history of smoking, irritating dry cough, bloody sputum, chest pain, emaciation, and changes in the initial characteristics of cough, lung carcinoma should be suspected. The diagnosis can be confirmed by radiological imaging and bronchoscopy biopsy (2D). Treatment for cough due to lung carcinoma should target at the underlying disease, including radiotherapy, chemotherapy, radiofrequency ablation, and surgical resection (371-373) (1A). Postoperative cough in patients with lung carcinoma is a common issue in clinical practice, and the underlying mechanisms are unclear. The cytokine inhibitor suplatast tosilate resolves cough (369,374) (2C). Protracted and refractory cough may be attenuated with the treatment of central-acting or peripheral-acting antitussives.
Psychogenic cough
Psychogenic cough, also known as habitual cough, is caused by severe psychological conditions. It is more common in children than in adults. Within the classifications of mental disorders, there is no diagnostic terminology for psychogenic cough, and the pathogenesis of psychological cough may involve not only psychological factors, but also disorders of central nervous system control. The term of somatic cough syndrome may be a better descriptor (375). Cough occurs only during the daytime, and disappears when focusing and when asleep. Multiple psychogenic factors such as sensation, belief, mood, learning, and habit can stimulate the cough, and these factors would be addressed in clinical practice (376).
There are no specific diagnostic criteria for psychogenic cough. When the common and rare causes of chronic cough are excluded, psychogenic cough should be considered. For the children with psychogenic cough, psychological intervention including suggestive therapy and psychological counseling may be beneficial (377-382) (2B). The short-term use of antitussives can be used as adjuvant therapy. For the adult patients, antianxiety or antidepressant medications, in combination with psychological interventions, may be helpful. In children, it is important to differentiate psychogenic cough from Tourette syndrome.
Other uncommon or rare causes of chronic cough
The uncommon and rare etiologies account only for a minor proportion of chronic cough, but involve a broad spectrum of conditions. Some uncommon or rare causes of chronic cough reported in the literatures are listed in Table 4.

Full table
Unexplained chronic cough, chronic cough hypersensitivity syndrome
In most patients with chronic cough, an etiologic diagnosis can be determined and cough can resolve after treatment. However, in some patients with chronic cough an etiologic diagnosis cannot be confirmed after a comprehensive investigation and therapy aimed at known causes. Traditionally, this type of cough is called unexplained cough, chronic refractory cough, or idiopathic cough. The diagnosis of unexplained chronic cough should include the following: all known causes of chronic cough have been excluded through systematic evaluation and patients fail to respond to etiology-targeted treatment. In these patients, many of whom are middle-aged women, triggered by acute infection of upper airways. In addition to chronic dry cough, patients have an itchy throat or an uncomfortable sensation in the throat. Patients are sensitive to smoke, dust, abnormal smells, and cold air, and sometimes talking or nervousness can induce coughing. Because the patients show the characters of cough hypersensitivity, a new diagnostic term, chronic CHS is now used to describe patients with this type of chronic cough (422).
Based on the pathophysiological characteristics of CHS, the treatment should be aimed to reduce the sensitivity of cough. However, therapeutic options for CHS including medicinal and non-medicinal treatment are limited. Clinical studies show that neuromodulators, for example gabapentin, are effective in the treatment of CHS (423) (2B). Other medicines including amitriptyline, baclofen, carbamazepine, and pregabalin may be useful in CHS (424) (2C). Other potential treatments including speech therapy, and cough suppression physical therapy. Cough suppression physical therapy improves the quality of life related to cough, cough hypersensitivity, and cough frequency (11,425-428) (2B).
Etiologic distribution and therapeutic principles for pediatric patients with chronic cough
A cough lasting for more than 4 weeks in children is defined as chronic cough which is different from that in adults. Cough may be the isolated or predominant symptom, with normal chest radiographic findings. The etiologic distribution of chronic cough in children is not identical to that in adults, and varies with age. For newborns and infants, congenital diseases, including tracheomalacia, supraglottic or glottic abnormalities, vascular malformations, primary ciliary dyskinesia, and bronchiectasis should be considered (429-437). For children younger than 3 years of age, airway infection should be initially considered (2C). The incidence of protracted bacterial bronchitis (PBB) is high in this age group, and half of the patients with PBB have tracheomalacia. Haemophilus influenzae is the causative bacteria in PBB (438-443) (2C). Bronchial alveolar lavage fluid (BALF) bacterial culture is useful to confirm the diagnosis. Airway foreign body aspiration is another important cause of chronic cough in children younger than 3 years of age. In children with a long history of cough but lack of response to routine treatment, the foreign body aspiration should be considered, and chest radiographs or bronchoscopy are required (444-448) (2C). Common causes of chronic cough in adults, PNDS and CVA, are not common in this age group. In children aged greater than 3 years, atopic diseases including asthma become common causes of chronic cough. In school-age children, CVA should be initially considered (2C). Upper airway cough syndrome can be caused by allergic rhinitis, rhinosinusitis, and adenoidal hypertrophy, and treatment is effective (438,442) (2C). EB is a common cause of chronic cough in adults; however, there have been no reports in pediatric patients. Causes of chronic cough that are rare in adults but common in pediatric patients include infections with atypical pathogens (mycoplasma and chlamydia), pertussis, foreign body aspiration, psychogenic cough, and congenital diseases. Due to physiological features, gastroesophageal reflux is a common phenomenon in healthy infants with an incidence of 40–65%. However, whether it is a common cause of cough in children remains uncertain.
Treatment principles for chronic cough in pediatric patients are to confirm the etiologic diagnosis and provide targeted treatment (2D). If the cause is unclear or relevant testing is not feasible because of age, empirical or symptomatic treatment can be implemented. If cough is not relieved after treatment, re-assessment should be performed. Antitussive agents should not be used for infants.
Empirical management of chronic cough
Successful treatment of chronic cough depends on etiologic diagnosis. Etiologic diagnosis may require specific equipment and techniques, which are not available in primary care hospitals, and are difficult for patients of low socioeconomic status to afford. Consequently, empirical treatment is an alternative in cases where conditions are limited (213,449-452) (2C). Empirical treatment should follow the principles below.
- Treatment schedules targeting the common causes of chronic cough are preferred. Many studies have referred that the common causes of chronic cough are CVA, UACS/PNDS, EB, AC and GERC (62,64,208,453) (1A).
- Possible causes of chronic cough should be identified based on the patient’s medical history (452,454) (2C). If nonproductive irritating cough is predominant symptom and cough frequently occur at night or early morning, initial treatment for CVA is recommended. If cough is concurrent with overt acid regurgitation, belching, and heartburn, treatment for GERC can be considered. If protracted cough is secondary to the common cold accompanied by runny nose, nasal congestion, nasal itching, frequent clearing of the throat, and postnasal drip, empirical treatment should initially target UACS/PNDS.
- According to the patient and treatment response, chronic cough can be classified into steroid-responsive cough (including CVA, EB and AC), UACS and GERS for empirical treatment. This may reduce the blindness of empirical therapy and increase the success rate (30) (2C). Patient-driven therapeutic strategy in a step-by-step and sequential manner (2C) is a diagnostic management regimen, which initially involves the most common causes and simple treatments. The treatment regimens may lead to a longer duration of therapy for patients with chronic cough of uncommon causes. The strategy is targeted at the most common causes first suitable for those patients with unclear characteristics and multiple possible causes of chronic cough (10,452,455-458) (1C). Pseudoephedrine hydrochloride and methoxyphenamine is recommended for the empirical treatment of UACS/PNDS, AC, and PIC (30) (2C). If steroid-responsive cough is suspected, oral administration of low-dose steroids for 1 week is recommended, followed by ICS or combined therapy with β2-receptor agonists (30) (2C).
- For those with purulent sputum or mucus nasal discharge, antibiotics are recommended (2D). Since most causes of chronic cough are not related to infection (62,208,453), antibiotic abuse should be avoided during empirical treatment.
- It is recommended that the course of empirical treatment for UACS, PNDS, CVA, and EB should be 1–2 weeks, and that for GERC should be 2–4 weeks (2D). Oral corticosteroids should be given for no more than 1 week (7). If the patient is responsive to the treatment, the standard therapeutic regimen for the corresponding etiology should be used.
- Empirical treatment may result in the risk of misdiagnosis of severe conditions. Empirical treatment should be used with caution, and serious conditions including bronchial malignant tumors, tuberculosis, and other pulmonary diseases should be ruled out. If empirical therapy is not effective, further investigations should be carried out to identify the etiologic diagnosis (10) (2D).
Cough suppressants and mucolytic agents
Mild cough does not require antitussive treatment. Antitussive agents can temporarily relieve the symptoms, and etiological therapy is the key to cough management. However, if severe dry or frequent cough impacts daily life, antitussive treatment can be prescribed. Mucolytic agents are indicated for patients with productive cough.
Antitussive agents
Antitussive agents can be divided into two types based on the pharmacologic effects: central-acting and peripheral-acting agents. Central-acting agents are those that act on one or several sites of cough center in the medulla oblongata, and peripheral-acting agents act on receptors on afferent or efferent nerves and effectors of the cough reflex arch (3,29,124).
Central-acting antitussive agents
These medications have inhibitory effects on the medulla oblongata. Based on the addiction potential and analgesic effects, they can be further subdivided into narcotic and non-narcotic antitussive agents. The former refers to morphine and its derivatives, which is a powerful cough suppressants. However, because of the possibility of addiction, they should only be used temporarily when other treatments failed. The latter refers to synthetic antitussive agents, such as dextromethorphan and pentoxyverine, which are widely used in clinical settings. (I) Narcotic antitussive agents: (i) Codeine (3): rapidly and directly inhibits the medulla oblongata and suppresses the cough, and has analgesic and sedative effects as usual. This type of medication can be used for patients who have unexplained severe dry cough or refractory irritating cough, in particular a dry cough with chest pain. (ii) Pholcodine: the effect is similar to codeine, but is less addictive. (II) Nonnarcotic antitussive agents: (i) dextromethorphan: it is similar in effect to codeine without analgesic and sedative effects. Therapeutic dosage usually does not have the inhibitory action on the respiratory center, nor side-effect related to medication addition. Dextromethorphan are recommended for chronic cough in adults (459) (2A). (ii) Pentoxyverine: cough suppressant strength is equal to one-third that of codeine with anticonvulsant and antispasmodic effects. It should be used cautiously in patients with glaucoma or heart failure. (iii) Dextrophan: a metabolite of dextromethorphan with better tolerance. It may replace dextromethorphan for clinical treatment in the future.
Peripheral-acting agents
They primarily act on inhibiting at least one element in the cough reflux arch. These drugs include local anesthetics and mucosal protectors. (I) Narcotine: it is a benzylisoquinolines alkaloid antitussive with effectiveness similar to codeine but without analgesic properties. It is suitable for cough induced by various causes. (II) Benproperine: it is a nonnarcotic drug with pharmaceutical effects 2–4 times greater than that of codeine. It inhibits both peripheral afferent nerves and the cough center. (III) Moguisteine: it is a peripheral non-narcotic antitussive drug, with a relatively stronger effect than that of codeine. (IV) Benzonatate: it is a local anesthetic that is a derivative of tetracaine and inhibits the afferent nerves of cough reflux.
Mucolytics
Mucolytics can improve clearance of airway secretions. The mechanisms of mucolytic agents include: increasing the clearance of secretions, decreasing the viscosity of secretions, and improving ciliary activity. There are a variety of mucolytics available, however, their effectiveness would require more evidence-based data. The common mucolytics are listed below.
Guaifenesin
It increases airway secretions and reduces sputum viscosity. It has been shown to cause bronchial dilation. It is used in combination with antihistamine, antitussive agents, and decongestants (460-462).
Myrtol
It is the extract of myrtaceae leaves, a volatile vegetable oil. The major components are eucalyptol, limonene, and alpha pinene. The commonly used preparations are eucalyptus and standard myrtol, which can improve the ciliary movement of the airway and nasal sinus mucosa, and are indicated for acute bronchitis, chronic bronchitis, and rhinosinusitis (463,464).
Ambroxol and bromhexine
These are mucolytic agents. Ambroxol is the metabolite of bromhexine, which can decompose the mucus acidic polysaccharide, decrease the viscosity of the secretion, improve ciliary activity, and increase the concentration of antibiotics in the respiratory tract.
N-acetylcysteine
It breaks down the sulfide bonds of the polypeptide chains of the glycoproteins to reduce the viscosity of sputum.
Carbocisteine
It breaks down the disulfide bonds of mucins to reduce the viscosity of secretion. Erdosteine is the precursor of carbocisteine. Oral administration can generate metabolites containing free sulfhydryl to exert pharmacological effect.
Others
Inhalation of hypertonic saline and mannitol can increase hydration of airway secretions, thus improving the rheology of mucus to enhance clearance. They can improve cough clearance in combined with bronchodilators (344,346).
TCM treatment
In TCM, cough is both a symptom and an independent disease. Chronic cough belongs to the category of “persistent cough” and “refractory cough”.
Cough was first mentioned by Huangdi’s Internal Classic over 2000 years ago, the classic also proposed that the etiology of cough involved all zang-fu organs, rather than just the lung-organ alone (465). Ancient Chinese classified cough by the internal zang-fu organs, which was similar to the anatomical classification of etiology in modern medicine. Cough has a variety of region-tailored types. It was summed up as exogenous and endogenous cough by Jingyue’s Complete Worksin, and this division is still used today (466). Briefly, cough (Ke Sou in TCM) results from lung qi failing in the purification and dispersion, along with lung qi ascending counter low.
With a long history, TCM has abundant experience in treating cough. In clinical practice, TCM treatment does relieve the cough of some patients with unexplained chronic cough. The advantages of TCM are as follows: giving treatment in accordance with seasonal conditions, local conditions and patient’s individuality, so as to offer accurate region-tailored treatment; administering multiple-link, multiple-target medications for therapeutic efficacy; following the principles of “symptomatic treatment in acute condition” and “radical treatment in chronic condition,” which is a comprehensive management model of “treating both principal and secondary aspect of disease”. There are many Chinese medicines, medical formulae, and prepared agents for cough treatment (467-469). A part of the common cough syndromes are listed below (468,470).
[Syndrome of lung Yin deficiency] Dry cough, minor sticky phlegm, or gradual hoarseness, dry mouth and throat, with a slow onset.
Methods of treatment: nourishing Yin and clearing heat; moistening the lung and relieving cough.
Examples of prescription: Decoction of Glehnia Ophiopogon (Treatise on Differentiation and Treatment of Warm Diseases), plus/minus: Radix Adenophorae, Radix Ophiopogonis, Radix polygonatum, Radix trichosanthis, white beans, mulberry leaves, Radix Glycyrrhizae.
[Syndrome of lung-kidney yang deficiency] Mild cough, occurring or worsening in cold environment, or accompanied with shortness of breath, soreness of waist and legs.
Methods of treatment: Tonifying lung and kidney; Warming Yang and relieving cough
Examples of prescription: Minor Blue Dragon Decoction (Treatise on Cold Damage Diseases) with Pills for Tonifying the Kidney Qi (“Synopsis of the Golden Chamber”), plus/minus: Ephedra, peony, Asarum, Ginger, cassia twig, Schisandra, Pinellia ternata, rehmannia, Chinese yam, Herba Epimedium, Morinda officinal, and licorice.
[Syndrome of stomach qi ascending counterflow] Paroxysmal irritating cough; severe cough causing vomiting of the gastric juice, aggravated when supine or after satiation; may be associated with belching with fetid odor and acids, noisy or burning, similar to gastroesophageal reflux-associated cough.
Methods of treatment: descending turbidity and resolving phlegm, harmonizing stomach and arresting the cough.
Examples of prescription: decoction of Inula and Hematite (Treatise on Cold Damage Diseases) with Pinellia Heart-Purging Decoction (Treatise on Cold Damage Diseases) plus/minus: Inula flower, ocher, ginseng, Pinellia, ginger, jujube, berberine, Scutellaria baicalensis, honey-fried licorice root.
[Syndrome of liver-fire attacking lung] Paroxysmal cough, with flushing of the face and eyes, and chest pain. Cough aggravation or remission associated with changes of mood; often with a sense of phlegm stagnation in the throat and difficulty in coughing out the phlegm; minor mucoid sputum, dry and bitter mouth.
Methods of treatment: clearing lung fire and purging heat; resolving phlegm and relieving cough.
Examples of prescription: powder of Scutellaria for Dispersing the lung (Zheng Yin Mai Zhi), with Natural Indigo and Clam Shell Powder (Chinese pharmacopoeia), plus/minus: Scutellaria baicalensis, cortex lycii radicis, Digupi, indigo naturalis, clam shell, and licorice.
[Syndrome of latent wind-pathogen tightening lung] Paroxysmal cough, accompanied by itchy throat, dry cough with minor sputum production, difficulty in sputum expectoration, often induced by cold air, odor, or laughing; without obvious chills and fever; external infection often induces cough aggravation or recurrence; pink tongue, with thin and white cover.
Methods of treatment: dispelling wind and dispersing lung; relieving cough and reducing phlegm.
Examples of prescription: Ephedra, folia perillae acutae, earthworm, folium eriobotryae, fructus perillae, periostracum cicada, Common hog fenneI root, burdock, Schisandra chinensis or Decoction of Three Crude Herbs (Bureau of Peaceful Benevolent Dispensary) with Cough-Stopping Powder (Understanding of Medicine), plus/minus broiled ephedra, almond, platycodon grandiflorum, herba schizonepetae, broiled asters, broiled radix stemonae, Cynanchum glaucescens, radix scutellariae, and radix glycyrrhizae.
[Syndrome of wind-cold invading lung] Severe cough, with itchy throat and shortness of breath; non-purulent sputum, nasal congestion, runny nose, headache, tongue with thin-white coating, pulse floating and tight.
Methods of treatment: expelling wind and cold; dispersing the lung and ceasing cough.
Prescription example: Cough-Stopping Powder (Understanding of Medicine) and Jade-Screen Powder (Jiu yuan Fang).
[Syndrome of wind-heat invading lung] Cough being frequent, with sore and painful throat; minor sputum expectoration, which is usually purulent; runny nose with purulent discharge, thirsty, headache, reddish tongue, the tongue with thin yellowish coating, pulse floating or frivolous.
Methods of treatment: dispelling wind and reducing heat; dispersing the lung and ceasing cough.
Examples of prescription: Powder of Honeysuckle and Forsythia (A Treatise on Differentiation and Treatment of Warm Diseases).
Concerning TCM, current treatment mainly based on some Chinese classical prescriptions, therapeutic methods, or the experience of senior experts. Due to the lack of definite evidence-based data, medical researches of TCM have a low evidence level (471). In China, there are a variety of prepared Chinese medicine compounds, but most of the treatments are staying at symptom management. The effective components, therapeutic indications, and adverse reactions are uncertain, and all of these need to be further investigated. Because of ambiguity of the differences between the “disease” and “syndrome”, the effective use of Chinese medicines is limited. Many Chinese patent medicine are indicated for syndromes, rather than for the specific etiology. In addition, it’s always not indicated whether this is antitussive or expectorant agent. Future medical research required TCM concepts combine with modern medical methods, to explore more Chinese Medicinal combination recipes and medicinal monomer with stronger efficacy and clearer indications.
Future developments
As a common problem in clinical practice, comprehensive studies on the etiologic diagnosis, treatment, and pathogenic mechanisms of chronic cough have been conducted for more than 30 years in Western countries and for almost 15 years in China. These efforts have resulted in a series of achievements. Experts from Europe, America, the United Kingdom, Japan, and Australia have developed guidelines for the management of cough. With in-depth studies of chronic cough and the application of guidelines, understanding the causes of chronic cough and the diagnosis and treatment level of Chinese health care providers has greatly improved. Some hospitals in China have specifically established cough labs for the induced sputum test, FeNO test and 24-h esophageal pH-multi-channel impedance monitoring. Furthermore, subspecialty and specialist outpatient clinics for chronic cough have been established in a lot of hospitals. Although there have been significant advances in the management of cough, clinicians are confronting challenges. First, as a common symptom in clinical practice, chronic cough has attracted more and more attention from clinicians. Nevertheless, a few institutes are currently involved in studying chronic cough in China. Therefore, we hope that more clinicians participate in the studies on chronic cough. Second, more clinicians have appreciated the diagnostic value and usefulness of the induced-sputum test for the assessment of responses to treatment. Although induced sputum tests have been increasingly applied in some hospitals in China, the clinical application is not sufficiently popular. Moreover, the challenges regarding the diagnosis, management, and pathogenesis of chronic cough merit further investigations, including the pathogenesis and treatment of protracted PIC; the mechanisms of UACS/PNDS; the relationship between pharyngeal diseases and UACS; the duration of treatment of EB/CVA and the relationship with classic asthma; the diagnostic criteria and pathogenesis of GERC; the correlation between air pollution and chronic cough; the therapy for unexplained cough or refractory cough; and the pathogenesis of cough hypersensitivity. As we look forward to the future, we realize we still have a long way to go. Only unremitting efforts will keep us moving further.
Supplementary
Supplementary file 1 Algorithm for the etiological diagnosis of chronic cough
Supplementary file 2 Hypertonic saline nebulization-induced sputum test
Sputum induction is performed using nebulization of hypertonic saline. The total and differential inflammatory cell counts may suggest the type and severity of bronchial inflammation, providing clues for the diagnosis and management of cough and prediction of the prognosis.
A single-concentration (concentration of NaCl: 3% or 4.5%) or a step-up concentration method (concentration of NaCl: 3%, 4%, and 5%) is used for hypertonic saline nebulization. In order to avoid adverse events, adults and children with severe asthma should receive normal saline nebulization when performing induced sputum test.
Reagents: hypertonic saline, 0.1% dithiothreitol (DTT), and hematoxylin-eosin or Swiss stains.
Instruments: ultrasonic or compressed nebulizer, water bath, whirlpool or horizontal oscillator, optical microscope.
Procedures:
- Hypertonic saline nebulization:
- Single-concentration method: (i) patients receive inhaled salbutamol (400 µg) 10 min before sputum induction; (ii) patients are asked to rinse their mouth with sterile water and blow their nose; (iii) after inhalation of hypertonic saline for 10 min, patients are asked to rinse mouth and blow the nose again, and cough up sputum into a sterile container; (iv) if the patient cannot cough up sputum or the amount of sputum is insufficient, step 3 will be repeated. The induction is terminated when a sufficient amount of sputum is obtained, or the total duration of nebulization reaches to 30 min. Step-up concentration method: (i) patients receive inhaled salbutamol (400 µg) 10 min before sputum induction; (ii) patients are asked to rinse their mouth with sterile water and blow their nose; (iii) after inhalation of aerosolized 3% hypertonic saline for 15 min, patients are asked to rinse mouth and blow their nose again, and cough up sputum into a sterile container; (iv) if the patient cannot cough up sputum or the amount of sputum is not sufficient, 4% hypertonic saline is used for nebulization for 8 min; (v) if patient still cannot cough up sputum or the amount of sputum is not sufficient, 5% hypertonic saline is used for nebulization for 7 min; (vi) if a sufficient amount of eligible sputum is obtained or the total duration of nebulization reaches to 30 min, sputum induction should be terminated.
- Sputum examination: Sputum sample should be processed shortly after expectoration, or preserved at 4 °C for less than 3 hrs. Sputum plugs should be picked up with forceps. The selected portion was fully mixed with 2 to 4 volumes of 0.1% DTT. After 10–15 min, the mixture should be filtrated with 48 µm filter or 300-mesh nylon gauze, and then centrifuged at 790 ×g under room temperature for 10 min. The dissolving effects of DTT can be potentiated in a 37 °C water bath and horizontal vortex. The supernatant will be removed whereas the cell pellets will be resuspended with normal saline. The total cell count is obtained with a Neubauer hemocytometer. A cell smear is prepared, and stained with hematoxylin-eosin or Swiss stain. Differential cell count is obtained by counting 400 non-squamous cells.
Precautions: (I) for patients with severe asthma or asthma exacerbation, the degree of airflow limitation determined by spirometry and clinical symptoms should be reviewed prior to determining if the patient is a suitable candidate for undergoing the induced sputum test. When the forced expiratory volume in one second (FEV1) is <60% of predicted value or in cases with obvious asthmatic symptoms, spontaneous cough-induced sputum or normal saline for nebulization is recommended. (II) The laboratory should be equipped with rescue protocols, instruments, and medications for adverse events such as acute exacerbation of asthma. During the induction period, the overall conditions of the patient and lung function should be closely monitored. (III) Steroids, antihistamines, and theophylline, which may affect the results, should be withheld for at least 3 days prior to the test. However, patients are allowed to continue anti-asthma medications if the test aims to assess the response to treatment and is conducted during the follow-up period.
Supplementary file 3 Multi-channel intraluminal impedance-pH monitoring (MII-pH)
Equipment: esophageal multi-channel impedance-pH monitoring device.
Procedures: (I) Prior to the procedure, calibrate the pH electrode in the buffer solutions at pH 4.0 and 7.0 (as specified by the manufacturer) to ensure the accuracy and stability of the instrument. (II) Select one nasal cavity for ease of passage of the catheter. After anesthetizing the nares with topical spray of 2% lidocaine, an esophageal manometry catheter is transnasally inserted into the esophagus. The location of lower esophageal sphincter is determined by the stationary pull-through technique. A combined catheter with six-impedance channel sensors and a pH electrode is used. The central positions of the impedance channel sensors are located at 3, 5, 7, 9, 15, and 17 cm above the lower esophageal sphincter. The pH electrode is positioned 5 cm above the manometrically located proximal border of the lower esophageal sphincter, with the external reference electrode fixed at a point in the middle-lower segment of sternum. The catheter is connected to a portable monitoring device that records and stores data from all seven channels (six impedance and one pH) with a frequency of 50 Hz.
Monitoring duration: over 18 hours.
Analysis of results: analysis includes the identification, enumeration, and classification of acid and non-acid reflux events, as well as the measurement and calculation of the parameters, including food bolus clearance, food bolus exposure, and esophageal acid clearance. A global measure of esophageal acid exposure is expressed by the DeMeester score, which is calculated by from the following six parameters: (I) total percentage of time with pH <4.0; (II) percentage of time with pH <4.0 when seated upright; (III) percentage of time with pH <4.0 when recumbent; (IV) the total number of reflux episodes; (V) the total number of reflux episodes lasting >5 min; and (VI) the duration of the longest reflux episode. Meanwhile, symptom-associated probability (SAP) is calculated to establish the temporal association between cough and the reflux detected.
Precautions: (I) fast for 10 hours before the test; (II) avoid acidic food in the meal prior to the examination; (III) stop antacids for 7 days, prokinetics for 3 days, and all these medications for 24 hours before examination. Maintain the treatment with these medications during the follow-up period observing the therapeutic efficacy; (IV) strictly and accurately record a diary of events; (V) during the monitoring period, maintain the usual daily lifestyle and do not limit the activities, but avoid acidic or spicy foods and beverages, as well as antacid medications.
Supplementary file 4 Cough provocation test
Cough is triggered by inhaling aerosolized capsaicin particles. A nebulizer is used to aerosolize capsaicin. Cough sensitivity is expressed as the cough threshold (C5), defined as the lowest concentration of capsaicin required for the induction of ≥5 coughs.
Reagent preparations: capsaicin (30.5 mg) is dissolved in 1 mL Tween 80 and 1 mL absolute ethanol, and mixed with 8 ml normal saline to yield a 0.01 mol/L stock solution. Prior to the test, the stock solution is serially diluted with normal saline into the following concentrations: 1.95, 3.9, 7.8, 15.6, 31.2, 62.5, 125, 250, 500, and 1,000 µmol/L.
Equipment: An inspiratory flow-driven dosimeter-controlled nebulizer is preferred. The flow velocity of compressed air is 0.11 L/s with a total output of 160 mg/min (normal saline as the control) and single inhalation time of 0.5 s. The subjects are instructed to gradually inhale from residual volume to total lung capacity. During the first half of inspiration, an aerosolized capsaicin solution is inhaled.
Procedures: (I) Normal saline is first inhaled as a negative control; (II) capsaicin solution is inhaled from the lowest concentration (1.95 µmol/L) for 30 s and the number of coughs that occur is recorded. If of the number of induced coughs do not meet C5, the next concentration of capsaicin is inhaled; (III) the test is terminated when ≥5 coughs are induced. The concentration of capsaicin represents the cough threshold C5. If after inhalation of the highest concentration (1,000 µmol/L) of capsaicin ≥5 coughs do not occur, the test should be discontinued, with the recorded cough threshold C5 of >1,000 µmol/L. If the subjects feel discomfort including severe heartburn, tachypnea, and dyspnea during the procedure, the test must be stopped immediately.
Precautions: (I) the solution used in the test should be prepared freshly; (II) contraindications: pregnancy, acute exacerbation of asthma, pneumothorax, recent hemoptysis, and severe heart diseases; (III) keep the subjects calm during the test. With the inhalation of capsaicin, avoid activities, such as speaking, which may influence the cough count.
Note: *, cannot be used as criteria for diagnosis, but can be used as an indicator for eosinophilic inflammation-related cough. (I) For patients with low economic status or for those in primary health care centers, empirical treatment can be adopted based on the medical history and cough-related symptoms. If the empirical treatment fails, to avoid the delay in proper diagnosis and therapy, patients should be referred to hospitals where the tests are available. (II) ACEI, angiotensin converting enzyme inhibitor; FeNO, Fractional exhaled nitric oxide; UACS, upper airway cough syndrome; PNDS: postnasal drip syndrome; CVA, cough variant asthma; EB, eosinophilic bronchitis; CT, computed tomography; Bronchoscopy, fiberoptic bronchoscopy; SPT, skin prick test; IgE, immunoglobulin E; GERC, gastroesophageal reflux-related cough; AC, atopic cough.
Acknowledgements
We would like to acknowledge all the members of this committee for their valuable contribution, consultants of guidelines revision, including Chunxue Bai, Minhu Chen, Ping Chen, Kehu Yang, Kaisheng Yin, Nanshan Zhong; peer review experts, including Yiqiang Chen, Yuanrong Dai, Shujuan Jiang, Yong Jiang, Lin Lin, Liqing Shi, Dejun Sun, Zhiqiang Sun, Changgui Wu, Feng Wu, Pusheng Xu, Xianwei Ye, Jia Zhu; as well as members contributed to literature searching, including Wuping Bao, Zhangfu Fang, Wen Hua, Junfeng Ji, Juan Li, Xiaoting Liang, Ruiyan Lin, Baojuan Liu, Jian Liu, Qianli Ma, Shengyun Shang, Xinming Su, Xiaoting Tang, Minghai Wu, Lixia Xia, Xianghuai Xu, Li Xu, Mengjie Yang, Shuang Yang, Xingmei Yu, Li Yu, Qiao Zhang, Weiyi Zhang, Yongming Zhang, Bonian Zhong. Our thanks also go to Weijie Guan, Xiaodong Liu, Shan Zhong, Wanyi Huang, Li Long, Wenzhi Zhan, for assistance in editing manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lai K, Li B, Wang F, et al. Survey on the diagnosis and management of the patients with chronic cough. Int J Respir 2011;31:645-7.
- Yang C, Chen R, Li B, et al. Survey of quality of life and incontinence in female patients with chronic cough. Int J Respir 2010;30:391-4.
- Dicpinigaitis PV, Morice AH, Birring SS, et al. Antitussive drugs—past, present, and future. Pharmacol Rev 2014;66:468-2. [Crossref] [PubMed]
- Lai K. Chinese National Guidelines on Diagnosis and Management of Cough: consensus and controversy. J Thorac Dis 2014;6:S683-8. [PubMed]
- Lai K, Pan J, Chen R, et al. Epidemiology of cough in relation to China. Cough 2013;9:18. [Crossref] [PubMed]
- Chinese Thoracic Society (CTS) Asthma Consortium, Clinical Practice Guidelines for Diagnosis and Managementof Cough. Zhonghua Jie He He Hu Xi Za Zhi 2005;28:738-44.
- Chinese Thoracic Society (CTS) Asthma Consortium, Clinical Practice Guidelines for Diagnosis and Management of Cough. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:407-13.
- Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S-3S. [Crossref] [PubMed]
- Morice AH, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004;24:481-2. [Crossref] [PubMed]
- Kohno S, Ishida T, Uchida Y, et al. The Japanese Respiratory Society guidelines for management of cough. Respirology 2006;11 Suppl 4:S135-6. [PubMed]
- Gibson PG, Chang AB, Glasgow NJ, et al. CICADA: Cough in Children and adults: Diagnosis and Assessment. Australian Cough Guidelines Summary Statement. Med J Aust 2010;192:265-71. [PubMed]
- McCrory DC, Lewis SZ. Methodology and grading of the evidence for the diagnosis and management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:28S-32S. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ 2008;336:1049-51. [Crossref] [PubMed]
- Jaeschke R, Guyatt GH, Dellinger P, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744. [Crossref] [PubMed]
- Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014;44:1149-55. [Crossref] [PubMed]
- Pan G, Zhang S, Feng Y, et al. Air pollution and Children's respiratory symptoms in six cities of Northern China. Respir Med 2010;104:1903-11. [Crossref] [PubMed]
- Lai HK, Ho SY, Wong CM, et al. Exposure to particulate air pollution at different living locations and respiratory symptoms in Hong Kong--an application of satellite information. Int J Environ Health Res 2010;20:219-30. [Crossref] [PubMed]
- Zhang JJ, Hu W, Wei F, et al. Children's respiratory morbidity prevalence in relation to air pollution in four Chinese cities. Environ Health Perspect 2002;110:961-7. [Crossref] [PubMed]
- Pierse N, Rushton L, Harris RS, et al. Locally generated particulate pollution and respiratory symptoms in young children. Thorax 2006;61:216-20. [Crossref] [PubMed]
- Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 2014;146:1633-48. [Crossref] [PubMed]
- Shi C, Qiu Z, Liu H, et al. The application of capsaicin cough challenge test in chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2007;30:954-6.
- Chen R, Luo W, Liu C, et al. A pilot study on normal reference value of capsaicin cough sensitivity. Int J Respir 2013;33:1334-7.
- Chen R, Luo W, Liu C, et al. Difference of Capsaicin Cough Sensitivity in Common Causes of Chronic Cough. Chin J Respir Crit Care Med 2013;12:384-9.
- Mazzone SB, Mclennan L, Mcgovern AE, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med 2007;176:327-32. [Crossref] [PubMed]
- Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid- in airway nerves of chronic cough. Am J Respir Crit Care Med 2004;170:1276-80. [Crossref] [PubMed]
- Grace M, Birrell MA, Dubuis E, et al. Transient receptor potential channels mediate the tussive response to prostaglandine2 and bradykinin. Thorax 2012;67:891-900. [Crossref] [PubMed]
- Birrell MA, Belvisi MG, Grace M, et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med 2009;180:1042-7. [Crossref] [PubMed]
- Benemei S, Patacchini R, Trevisani M, et al. TRP channels. Curr Opin Pharmacol 2015;22:18-23. [Crossref] [PubMed]
- Lai K. Chronic Cough. People’s Medical Publishing House, 2008.
- Deng HY, Luo W, Zhang M, et al. Initial empirical treatment based on clinical feature of chronic cough. Clin Respir J 2016;10:622-30. [Crossref] [PubMed]
- Xi Y, Lai K, Chen R, et al. Clinical characteristics of cough variant asthma and it's relationship with the classic asthma. Chin J Asthma (Electronic Version) 2011;(3):1-6.
- Lai KF, Chen RC, Lin L, et al. Diagnostic values of the clinical characteristics of chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:418-21. [PubMed]
- Liu CL, Lai KF, Chen RC, et al. The clinical features and the diagnosis of gastro-esophageal reflux induced cough. Zhonghua Nei Ke Za Zhi 2005;44:438-41. [PubMed]
- Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult Sinusitis Executive Summary. Otolaryngol Head Neck Surg 2015;152:598-609. [Crossref] [PubMed]
- Yu X, Zhu H, Hao C, et al. Characteristics of airway hyperresponsiveness in children with chronic cough of different causes. Zhonghua Jie He He Hu Xi Za Zhi 2015;38:55-8. [PubMed]
- Wang Z, Li Y, Huang T, et al. Diagnostic value of bronchial provocation test in patients with chronic cough. Chin J Respir Crit Care Med 2007;6:245-8.
- Lai KF, Chen RC, Liu CL, et al. Etiology and a diagnostic protocol for patients with chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2006;29:96-9. [PubMed]
- Sano T, Ueda H, Bando H. A preliminary study of PEFR monitoring in patients with chronic cough. Lung 2004;182:285-95. [Crossref] [PubMed]
- Nakade Y, Fujimura M, Ohkura N, et al. Reversibility of the pulmonary function based on the partial flow-volume curve predicts the efficacy of bronchodilator therapy for treating chronic cough. Intern Med 2013;52:2017-23. [Crossref] [PubMed]
- Luo W, Chen R, Liu C, et al. Diagnostic significance of differential cell count in induced sputum to chronic cough. Chin J Lab Med 2007;30:280-3.
- Nair P, Hargreave FE. Measuring bronchitis in airway diseases: clinical implementation and application: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 2010;138:38S-43S. [Crossref] [PubMed]
- Moritz P, Steidle LJ, Felisbino MB, et al. Determination of the inflammatory component of airway diseases by induced sputum cell counts: use in clinical practice. J Bras Pneumol 2008;34:913-21. [Crossref] [PubMed]
- Brightling CE. Clinical applications of induced sputum. Chest 2006;129:1344-8. [Crossref] [PubMed]
- Luo W, Wan GH, Chen R. Comparative study on the success rate and safety of sputum induction with hypertonic saline nebulization with single concentration and increasing concentration. Guangdong Medical Journal 2010;31:3193-5.
- van der Vaart H, Postm AD, Timens W, et al. Repeated sputum inductions induce a transient neutrophilic and eosinophilic response. Chest 2006;130:1157-64. [Crossref] [PubMed]
- James AL, Maxwell PS, Elliot JG. Comparison of sputum induction using inhaled methacholine or hypertonic saline. Respirology 2005;10:57-62. [Crossref] [PubMed]
- Zhang YM, Lin JT, Su N, et al. Values of fractional exhaled nitric oxide in the diagnosis of chronic cough. Zhonghua Yi Xue Za Zhi 2011;91:1254-8. [PubMed]
- Zhang YM, Lin JT. The values of fractional exhaled nitric oxide in the diagnosis and treatment of chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2011;34:504-8. [PubMed]
- Westerhof GA, Korevaar DA, Amelink M, et al. Biomarkers to identify sputum eosinophilia in different adult asthma phenotypes. Eur Respir J 2015;46:688-96. [Crossref] [PubMed]
- Sato S, Saito J, Sato Y, et al. Clinical usefulness of fractional exhaled nitric oxide for diagnosing Prolonged cough. Respir Med 2008;102:1452-9. [Crossref] [PubMed]
- Pacheco A, Faro V, Cobeta I, et al. Gastro-oesophageal reflux, eosinophilic airway inflammation and chronic cough. Respirology 2011;16:994-9. [Crossref] [PubMed]
- Oh MJ, Lee JY, Lee BJ, et al. Exhaled nitric oxide measurement is useful for the exclusion of nonasthmatic eosinophilic bronchitis in patients with chronic cough. Chest 2008;134:990-5. [Crossref] [PubMed]
- Kowal K, Bodzenta-Lukaszyk A, Zukowski S. Exhaled nitric oxide in evaluation of young adults with chronic cough. J Asthma 2009;46:692-8. [Crossref] [PubMed]
- Yi F, Chen R, Luo W, et al. Validity of fractional exhaled nitric oxide in diagnosis of corticosteroids responsive cough. Chest 2016;149:1042-51. [Crossref] [PubMed]
- Soter S, Barta I, Antu SB. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation 2013;36:1178-85. [Crossref] [PubMed]
- Alvarez-Puebla MJ, Olaguibel Rivera JM, Almudevar E, et al. Cutoff point for exhaled nitric oxide corresponding to 3% sputum eosinophils. J Investig Allergol Clin Immunol 2015;25:107-11. [PubMed]
- Lai K, Liu B, Xu D, et al. Will nonasthmatic eosinophilic bronchitis develop into chronic airway obstruction?: A Prospective, Observational Study. Chest 2015;148:887-94. [Crossref] [PubMed]
- Chinese Thoracici Society. Diagnostic Flexible Bronchoscopy Application Guidelines. Zhonghua Jie He He Hu Xi Za Zhi 2008;1:14-7.
- Tian J, Lai K, Yi F, et al. A case of tracheal adenoid cystic carcinoma presented with chronic cough. Chinese Journal for Clinicians 2014;7:93-4.
- Decalmer S, Woodcock A, Greaves M, et al. Airway abnormalities at flexible bronchoscopy in patients with chronic cough. Eur Respir J 2007;30:1138-42. [Crossref] [PubMed]
- Barnes TW, Afessa B, Swanson KL, et al. The clinical utility of flexible bronchoscopy in the evaluation of chronic cough. Chest 2004;126:268-72. [Crossref] [PubMed]
- Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013;143:613-20. [Crossref] [PubMed]
- Luo W, Lai K, Chen R, et al. Establishment of reference values for cellularity in induced sputum of healthy adults in Guangzhou. Int J Respir 2007;27:1213-5.
- Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis 1981;123:413-7. [PubMed]
- Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med 1999;160:406-10. [Crossref] [PubMed]
- Ma HM, Zhu LX, Lai KF, et al. Etiological diagnosis of chronic cough with unknown causes. Zhonghua Jie He He Hu Xi Za Zhi 2003;26:675-8. [PubMed]
- Lu GL, Lin JT. The spectrum and clinical features of causes for chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:422-5. [PubMed]
- Cao G, Cheng X, Dai X, et al. A Multi-Center Study on Clinical and Etiological Diagnosis of Chronic Cough in Chongqing City. Chin J Respir Crit Care Med 2009;8:565-8.
- Si S, Peng Q, Shi X, et al. The etiology and clinical characteristics of chronic cough in Shenyang city and surrounding areas. Zhonghua Jie He He Hu Xi Za Zhi 2010.862-3.
- Chung KF. Measurement of cough. Respir Physiol Neurobiol 2006;152:329-39. [Crossref] [PubMed]
- Irwin RS. Assessing cough severity and efficacy of therapy in clinical research: ACCP evidence-based clinical practice guidelines. Chest 2006;129:232S-237S. [Crossref] [PubMed]
- Shi X, Peng QF, Kong LF. An analysis of factors for quality-of-life in patients with chronic cough. Zhonghua Nei Ke Za Zhi 2011;50:672-5. [PubMed]
- Yousaf N, Lee KK, Jayaraman B, et al. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 2011;7:4. [Crossref] [PubMed]
- Spinou A, Birring SS. An update on measurement and monitoring of cough: what are the important study endpoints? J Thorac Dis 2014;6:S728-34. [PubMed]
- Schmit KM, Coeytaux RR, Goode AP, et al. Evaluating cough assessment tools: a systematic review. Chest 2013;144:1819-26. [Crossref] [PubMed]
- Murray MP, Turnbull K, Macquarrie S, et al. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009;34:125-31. [Crossref] [PubMed]
- Ma W, Yu L, Wang Y, et al. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough 2009;5:7. [Crossref] [PubMed]
- Leconte S, Ferrant D, Dory V, et al. Validated methods of cough assessment: a systematic review of the literature. Respiration 2011;81:161-4. [Crossref] [PubMed]
- French CT, Fletcher KE, Irwin RS. A comparison of gender differences in health-related quality of life in acute and chronic coughers. Chest 2005;127:1991-8. [Crossref] [PubMed]
- Birring SS, Pavord ID. Assessment of gender differences in health status with the Leicester Cough Questionnaire (LCQ). Thorax 2009;64:1008-9. [Crossref] [PubMed]
- Berkhof FF, Boom LN, Ten Hertog NE, et al. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes 2012;10:4. [Crossref] [PubMed]
- Barry SJ, Dane AD, Morice AH, et al. The automatic recognition and counting of cough. Cough 2006;2:8. [Crossref] [PubMed]
- Matos S, Birring SS, Pavord ID, et al. An automated system for 24-h monitoring of cough frequency: the leicester cough monitor. IEEE Trans Biomed Eng 2007;54:1472-9. [Crossref] [PubMed]
- Ma X, Zheng Z, Chen R, et al. Application of speech recognition technology in the automatic recognition of cough sounds. Chin J Biomed Eng 2010;16:564-6.
- Ma QL, Long Z, Zhang Q, et al. The significance of cough reflex sensitivity test in chronic cough. Zhonghua Nei Ke Za Zhi 2011;50:668-71. [PubMed]
- Decalmer SC, Webster D, Kelsall A A, et al. Chronic cough: how do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax 2007;62:329-34. [Crossref] [PubMed]
- Dicpinigaitis PV, Alva RV. Safety of capsaicin cough challenge testing. Chest 2005;128:196-202. [Crossref] [PubMed]
- Morice AH, Mcgarvey L, Pavord I, et al. Recommendations for the management of cough in adults. Thorax 2006;61 Suppl 1:i1-4. [Crossref] [PubMed]
- Chen RC, Lai KF, Liu CL, et al. The development and safety of cough provocation test by capsaicin inhalation. Zhonghua Jie He He Hu Xi Za Zhi 2005;28:751-4. [PubMed]
- Varechova S, Plevkova J, Hanacek J, et al. Role of gender and pubertal stage on cough sensitivity in childhood and adolescence. J Physiol Pharmacol 2008;59 Suppl 6:719-26. [PubMed]
- Kelsall A, Decalmer S, McGuinness K, et al. Sex differences and predictors of objective cough frequency in chronic cough. Thorax 2009;64:393-8. [Crossref] [PubMed]
- Xiong C, Cheng X. Early identification and treatment of acute pulmonary embolism. Chin J Gen Pract 2003;2:80-2.
- Wen W, Zhang X. Clinical characteristics of acute pulmonary embolism in 50 elderly patients. Chin J Geriatr 2006;25:431-3.
- Huang S. Diagnosis of acute cough. Chinese Journal of Practical Internal Medicine 2006;26:1325-7.
- Leuzzi G, Kawamukai K, Lacava N. An unusual foreign body after dental filling. Lung 2013;191:677-8. [Crossref] [PubMed]
- Holzinger F, Beck S, Dini L, et al. The Diagnosis and Treatment of Acute Cough in Adults. Deutsches Arzteblatt International 2014;111:356-63. [PubMed]
- Bulgiba AM, Razaz M. How well can signs and symptoms predict AMI in the Malaysian population? Int J Cardiol 2005;102:87-93. [Crossref] [PubMed]
- Zimmerman RK, Rinaldo CR, Nowalk MP, et al. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011-2 influenza season. Influenza Other Respir Viruses 2014;8:397-405. [Crossref] [PubMed]
- Sundaram ME, Meece JK, Sifakis F, et al. Medically attended respiratory syncytial virus infections in adults aged >/= 50 years: clinical characteristics and outcomes. Clin Infect Dis 2014;58:342-9. [Crossref] [PubMed]
- Chinese Medical Doctor Association Respir Med Branch & Emergency Medicine Branch. Expert consensus on management of common cold. Chin J Crit Care Med 2012;32:961-5.
- Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis 2005;5:718-25. [Crossref] [PubMed]
- Cedraschi C, Saya L, Klein P, et al. Representations of influenza and influenza-like illness in the community--a qualitative study. BMC Fam Pract 2013;14:15. [Crossref] [PubMed]
- Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med 2009;361:2507-17. [Crossref] [PubMed]
- Lin L, Yang ZF, Zhan YQ, et al. The duration of cough in patients with H1N1 influenza. Clin Respir J 2017;11:733-8. [Crossref] [PubMed]
- Wong DM, Blumberg DA, Lowe LG. Guidelines for the use of antibiotics in acute upper respiratory tract infections. Am Fam Physician 2006;74:956-66. [PubMed]
- Tomii K, Matsumura Y, Maeda K, et al. Minimal use of antibiotics for acute respiratory tract infections: validity and patient satisfaction. Intern Med 2007;46:267-72. [Crossref] [PubMed]
- Spurling GK, Del Mar CB, Dooley L, et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev 2013;4:CD004417. [PubMed]
- Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev 2013;6:CD000247. [PubMed]
- Arroll B. Antibiotics for upper respiratory tract infections: an overview of Cochran e reviews. Respir Med 2005;99:255-61. [Crossref] [PubMed]
- Wiest E, Jones JS. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 1: use of non-sedating antihistamines in the common cold. Emerg Med J 2011;28:632-3. [PubMed]
- Dealleaume L, Tweed B, Neher JO. Do OTC remedies relieve cough in acute URIs? J Fam Pract 2009;58:559a-c. [PubMed]
- De Sutter AI, Lemiengre M, Campbell H. WITHDRAWN: Antihistamines for the common cold. Cochrane Database Syst Rev 2009.CD001267. [PubMed]
- Arroll B. Non-antibiotic treatments for upper-respiratory tract infections (common cold). Respir Med 2005;99:1477-84. [Crossref] [PubMed]
- Taverner D, Latte J. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2007.CD001953. [PubMed]
- Latte J, Taverner D. Clinical trial of 3 days of treatment with oral pseudoephedrine for the common cold in the southern hemisphere. Am J Rhinol 2007;21:452-5. [Crossref] [PubMed]
- Eccles R, Jawad MS, Jawad SS, et al. Efficacy and safety of single and multiple doses of pseudoephedrine in the treatment of nasal congestion associated with common cold. Am J Rhinol 2005;19:25-31. [Crossref] [PubMed]
- Yin Y, Zhu J, Li L, et al. The efficacy of acetaminophen on the treatment of 151 patients with common cold. Chin J Crit Care Med 2007;16:1201-2.
- Schachtel BP, Voelker M, Sanner KM, et al. Demonstration of the analgesic efficacy and dose-response of acetylsalicylic acid with pseudoephedrine. J Clin Pharmacol 2010;50:1429-37. [Crossref] [PubMed]
- Loose I, Winkel M. Clinical, double-blind, placebo-controlled study investigating the combination of acetylsalicylic acid and pseudoephedrine for the symptomatic treatment of nasal congestion associated with common cold. Arzneimittelforschung 2004;54:513-21. [PubMed]
- Li S, Yue J, Dong BR, et al. Acetaminophen (paracetamol) for the common cold in adults. Cochrane Database Syst Rev 2013;7:CD008800. [PubMed]
- Kim SY, Chang YJ, Cho HM, et al. Non-steroidal anti-inflammatory drugs for the common cold. Cochrane Database Syst Rev 2013;6:CD006362. [PubMed]
- Goto M, Kawamura T, Shimbo T, et al. Influence of loxoprofen use on recovery from naturally acquired upper respiratory tract infections: a randomized controlled trial. Intern Med 2007;46:1179-86. [Crossref] [PubMed]
- Eccles R, Jawad M, Jawad S, et al. Efficacy of a paracetamol-pseudoephedrine combination for treatment of nasal congestion and pain-related symptoms in upper respiratory tract infection. Curr Med Res Opin 2006;22:2411-8. [Crossref] [PubMed]
- Bolser DC. Cough suppressant and pharmacologic protussive therapy: ACCP evidence-based clinical practice guidelines. Chest 2006;129:238S-249S. [Crossref] [PubMed]
- Qu W, Liu H, Zhao L, et al. A multicenter randomized double blind parallel clinical trial of compound methylephedrine hydrochloride capsules in the treatment of common cold. The Chinese Journal of Clinical Pharmacology 2011;27:83-5,91.
- Ji R, Cao Z, He Q, et al. A Randomized controlled Multicenter Clinical Trial on The Treatment of Common Cold with Fu-fang-an-fen-jia-ma Fluid. The Chinese Journal of Clinical Pharmacology 2003;19:167-70.
- Picon PD, Costa MB, Da Veiga Picon R, et al. Symptomatic treatment of the common cold with a fixed-dose combination of paracetamol, chlorphenamine and phenylephrine: a randomized, placebo-controlled trial. BMC Infect Dis 2013;13:556. [Crossref] [PubMed]
- Mizoguchi H, Wilson A, Jerdack GR, et al. Efficacy of a single evening dose of syrup containing paracetamol, dextromethorphan hydrobromide, doxylamine succinate and ephedrine sulfate in subjects with multiple common cold symptoms. Int J Clin Pharmacol Ther 2007;45:230-6. [Crossref] [PubMed]
- De Sutter AI, Van Driel ML, Kumar AA, et al. Oral antihistamine-decongestant-analgesic combinations for the common cold. Cochrane Database Syst Rev 2012;2:CD004976. [PubMed]
- Common Cold Collaborative Group, Robert M, Llorens M, et al. Efficacy and tolerability of ebastine 10 mg plus pseudoephedrine 120 mg in the symptomatic relief of the common cold. Eur J Intern Med 2004;15:242-7. [Crossref] [PubMed]
- Eccles R, Pedersen A, Regberg D, et al. Efficacy and safety of topical combinations of ipratropium and xylometazoline for the treatment of symptoms of runny nose and nasal congestion associated with acute upper respiratory tract infection. Am J Rhinol 2007;21:40-5. [Crossref] [PubMed]
- AlBalawi ZH, Othman SS, Alfaleh K. Intranasal ipratropium bromide for the common cold. Cochrane Database Syst Rev 2013;6:CD008231. [PubMed]
- Wu T, Zhang J, Qiu Y, et al. Chinese medicinal herbs for the common cold. Cochrane Database Syst Rev 2007.CD004782. [PubMed]
- Timmer A, Gunther J, Motschall E, et al. Pelargonium sidoides extract for treating acute respiratory tract infections. Cochrane Database Syst Rev 2013;10:CD006323. [PubMed]
- Zhou Y, Lu X, Chen X, et al. Viral Etiology of Acute Lower Respiratory Infection in Adult Inpatients. Chin J Respir Crit Care Med 2010;9:378-82.
- Wenzel RP, Fowler AA 3rd. Clinical practice. Acute bronchitis. N Engl J Med 2006;355:2125-30. [Crossref] [PubMed]
- van Gageldonk-Lafeber AB, Heijnen ML, Bartelds AI, et al. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis 2005;41:490-7. [Crossref] [PubMed]
- Ren L, Gonzalez R, Wang Z, et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005-007. Clin Microbiol Infect 2009;15:1146-53. [Crossref] [PubMed]
- Ikematsu H, Takeuchi Y, Rosenlund M, et al. The post-infection outcomes of influenza and acute respiratory infection in patients above 50 years of age in Japan: an observational study. Influenza Other Respir Viruses 2012;6:211-7. [Crossref] [PubMed]
- Gencay M, Roth M, Christ-Crain M, et al. Single and multiple viral infections in lower respiratory tract infection. Respiration 2010;80:560-7. [Crossref] [PubMed]
- Creer DD, Dilworth JP, Gillespie SH, et al. Aetiological role of viral and bacterial infections in acute adult lower respiratory tract infection (LRTI) in primary care. Thorax 2006;61:75-9. [Crossref] [PubMed]
- Worrall G. Acute bronchitis. Can Fam Physician 2008;54:238-9. [PubMed]
- Altiner A, Wilm S, Daubener W, et al. Sputum colour for diagnosis of a bacterial infection in patients with acute cough. Scand J Prim Health Care 2009;27:70-3. [Crossref] [PubMed]
- Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician 2010;82:1345-50. [PubMed]
- Wark P. Bronchitis (acute). BMJ Clin Evid 2011;2011.
- Tackett KL, Atkins A. Evidence-based acute bronchitis therapy. J Pharm Pract 2012;25:586-90. [Crossref] [PubMed]
- Hart AM. Evidence-based diagnosis and management of acute bronchitis. Nurse Pract 2014;39:32-39. [Crossref] [PubMed]
- Ebell MH, Lundgren J, Youngpairoj S. How long does a cough last? Comparing patients' expectations with data from a systematic review of the literature. Ann Fam Med 2013;11:5-13. [Crossref] [PubMed]
- Blush RR 3rd. Acute bronchitis: evaluation and management. Nurse Pract 2013;38:14-20. [Crossref] [PubMed]
- Nolt BR, Gonzales R, Maselli J, et al. Vital-sign abnormalities as predictors of pneumonia in adults with acute cough illness. Am J Emerg Med 2007;25:631-6. [Crossref] [PubMed]
- Evertsen J, Baumgardner DJ, Regnery A, et al. Diagnosis and management of pneumonia and bronchitis in outpatient primary care practices. Prim Care Respir J 2010;19:237-41. [Crossref] [PubMed]
- Aagaard E, Maselli J, Gonzales R. Physician practice patterns: chest x-ray ordering for the evaluation of acute cough illness in adults. Med Decis Making 2006;26:599-605. [Crossref] [PubMed]
- Zanasi A, Mazzolini M, Tursi F, et al. Homeopathic medicine for acute cough in upper respiratory tract infections and acute bronchitis: a randomized, double-blind, placebo-controlled trial. Pulm Pharmacol Ther 2014;27:102-8. [Crossref] [PubMed]
- Schulz M, Hammerlein A, Hinkel U, et al. Safety and usage pattern of an over-the-counter ambroxol cough syrup: a community pharmacy-based cohort study. Int J Clin Pharmacol Ther 2006;44:409-21. [Crossref] [PubMed]
- Prabhu Shankar S, Chandrashekharan S, Bolmall CS, et al. Efficacy, safety and tolerability of salbutamol + guaiphenesin + bromhexine (Ascoril) expectorant versus expectorants containing salbutamol and either guaiphenesin or bromhexine in productive cough: a randomised controlled comparative study. J Indian Med Assoc 2010;108:313-314, 316-318, 320. [PubMed]
- Hoffer-Schaefer A, Rozycki HJ, Yopp MA, et al. Guaifenesin has no effect on sputum volume or sputum properties in adolescents and adults with acute respiratory tract infections. Respir Care 2014;59:631-6. [Crossref] [PubMed]
- Gillissen A, Wittig T, Ehmen M, et al. A multi-centre, randomised, double-blind, placebo-controlled clinical trial on the efficacy and tolerability of GeloMyrtol(R) forte in acute bronchitis. Drug Res (Stuttg) 2013;63:19-27. [Crossref] [PubMed]
- Fischer J, Dethlefsen U. Efficacy of cineole in patients suffering from acute bronchitis: a placebo-controlled double-blind trial. Cough 2013;9:25. [Crossref] [PubMed]
- Wang J, Wang P, Wang X, et al. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med 2014;174:1914-20. [Crossref] [PubMed]
- Treebupachatsakul P, Tiengrim S, Thamlikitkul V. Upper respiratory tract infection in Thai adults: prevalence and prediction of bacterial causes, and effectiveness of using clinical practice guidelines. J Med Assoc Thai 2006;89:1178-86. [PubMed]
- Smith SM, Fahey T, Smucny J, et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2014;3:CD000245. [PubMed]
- Nduba VN, Mwachari CW, Magaret AS, et al. Placebo found equivalent to amoxicillin for treatment of acute bronchitis in Nairobi, Kenya: a triple blind, randomised, equivalence trial. Thorax 2008;63:999-1005. [Crossref] [PubMed]
- Llor C, Moragas A, Bayona C, et al. Efficacy of anti-inflammatory or antibiotic treatment in patients with non-complicated acute bronchitis and discoloured sputum: randomised placebo controlled trial. BMJ 2013;347:f5762. [Crossref] [PubMed]
- Llor C, Moragas A, Bayona C, et al. Effectiveness of anti-inflammatory treatment versus antibiotic therapy and placebo for patients with non-complicated acute bronchitis with purulent sputum. The BAAP study protocol. BMC Pulm Med 2011;11:38. [Crossref] [PubMed]
- Little P, Stuart B, Moore M, et al. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infect Dis 2013;13:123-9. [Crossref] [PubMed]
- Laopaiboon M, Panpanich R, Swa Mya K. Azithromycin for acute lower respiratory tract infections. Cochrane Database Syst Rev 2015;3:CD001954. [PubMed]
- Coenen S, Van Royen P, Michiels B, et al. Optimizing antibiotic prescribing for acute cough in general practice: a cluster-randomized controlled trial. J Antimicrob Chemother 2004;54:661-2. [Crossref] [PubMed]
- Phillips TG, Hickner J. Calling acute bronchitis a chest cold may improve patient satisfaction with appropriate antibiotic use. J Am Board Fam Pract 2005;18:459-63. [Crossref] [PubMed]
- Ong S, Nakase J, Moran GJ, et al. Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction. Ann Emerg Med 2007;50:213-20. [Crossref] [PubMed]
- Manoharan A, Winter J. Tackling upper respiratory tract infections. Practitioner 2010;254:25-8, 2-3.
- Kroening-Roche JC, Soroudi A, Castillo EM, et al. Antibiotic and bronchodilator prescribing for acute bronchitis in the emergency department. J Emerg Med 2012;43:221-7. [Crossref] [PubMed]
- Eilat-Tsanani S, Tabenkin H, Chazan B, et al. Acute cough: the use of antibiotics and health care services in an urban health centre in Israel. Eur J Gen Pract 2013;19:92-8. [Crossref] [PubMed]
- Coenen S, Michiels B, Renard D, et al. Antibiotic prescribing for acute cough: the effect of perceived patient demand. Br J Gen Pract 2006;56:183-90. [PubMed]
- Cals JW, Boumans D, Lardinois RJ, et al. Public beliefs on antibiotics and respiratory tract infections: an internet-based questionnaire study. Br J Gen Pract 2007;57:942-7. [Crossref] [PubMed]
- Becker LA, Hom J, Villasis-Keever M, et al. Beta2-agonists for acute bronchitis. Cochrane Database Syst Rev 2011.CD001726. [PubMed]
- Wu T, Yang X, Zeng X, et al. Traditional Chinese medicine in the treatment of acute respiratory tract infections. Respir Med 2008;102:1093-8. [Crossref] [PubMed]
- Jiang L, Li K, Wu T. Chinese medicinal herbs for acute bronchitis. Cochrane Database Syst Rev 2012;2:CD004560. [PubMed]
- Lai K, Lin L, Liu B, et al. Eosinophilic airway inflammation is common in subacute cough following acute upper respiratory tract infection. Respirology 2016;21:683-8. [Crossref] [PubMed]
- Kwon NH, Oh MJ, Min TH, et al. Causes and clinical features of subacute cough. Chest 2006;129:1142-7. [Crossref] [PubMed]
- Ryan NM, Vertigan AE, Ferguson J, et al. Clinical and physiological features of postinfectious chronic cough associated with H1N1 infection. Respir Med 2012;106:138-44. [Crossref] [PubMed]
- Kardos P, Berck H, Fuchs KH, et al. Guidelines of the German Respiratory Society for diagnosis and treatment of adults suffering from acute or chronic cough. Pneumologie 2010;64:701-11. [Crossref] [PubMed]
- Zhou X, Bao W, Qu J, et al. A multicenter study to evaluate the efficacy and safety of compound methoxyphenamine in the treatment of postinfectious cough. Int J Respir 2011;31:1761-5.
- Wang K, Birring SS, Taylor K, et al. Montelukast for postinfectious cough in adults: a double-blind randomised placebo-controlled trial. Lancet Respir Med 2014;2:35-43. [Crossref] [PubMed]
- Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev 2013;3:CD009305. [PubMed]
- Gillissen A, Richter A, Oster H. Clinical efficacy of short-term treatment with extra-fine HFA beclomethasone dipropionate in patients with post-infectious persistent cough. J Physiol Pharmacol 2007;58 Suppl 5:223-32. [PubMed]
- Zhang YP, Cao E, Miao Q, et al. A Randomized Controlled Study on Suhuang Zhike Capsule for Treatment of Post-cold Cough. Chinese Journal of Integrated Traditional and Western Medicine 2008;28:698-701. [PubMed]
- Clinical Research Coordination Group of the Causes Constituents Ratio of Chronic Cough in Chinese Children. A prospective multicenter clinical study on the causes constituents ratio of chronic cough in Chinese children. Chinese Journal of Pediatrics 2012;50:83-92. [PubMed]
- Yuan X, Liu Y, Bai C, et al. Mycoplasma pneumoniae infection is associated with subacute cough. Eur Respir J 2014;43:1178-81. [Crossref] [PubMed]
- Hare KM, Marsh RL, Smith-Vaughan HC, et al. Respiratory bacterial culture from two sequential bronchoalveolar lavages of the same lobe in children with chronic cough. J Med Microbiol 2015;64:1353-60. [Crossref] [PubMed]
- Uehara S, Sunakawa K, Eguchi H, et al. Japanese Guidelines for the Management of Respiratory Infectious Diseases in Children 2007 with focus on pneumonia. Pediatr Int 2011;53:264-6. [Crossref] [PubMed]
- Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect 2003;9:263-73. [Crossref] [PubMed]
- Chang AB, Oppenheimer JJ, Weinberger M, et al. Children with chronic wet or productive cough - treatment and investigations: a systematic review. Chest 2016;149:120-42. [Crossref] [PubMed]
- Marchant J, Masters IB, Champion A, et al. Randomised controlled trial of amoxycillin clavulanate in children with chronic wet cough. Thorax 2012;67:689-93. [Crossref] [PubMed]
- Kapaskelis AM, Vouloumanou EK, Rafailidis PI, et al. High prevalence of antibody titers against Bordetella pertussis in an adult population with prolonged cough. Respir Med 2008;102:1586-91. [Crossref] [PubMed]
- Hu JJ, Lu CY, Chang LY, et al. Survey of pertussis in patients with prolonged cough. J Microbiol Immunol Infect 2006;39:54-8. [PubMed]
- Dalby T, Harboe ZB, Krogfelt KA. Seroprevalence of pertussis among Danish patients with cough of unknown etiology. Clin Vaccine Immunol 2010;17:2016-23. [Crossref] [PubMed]
- Rutledge RK, Keen EC. Images in clinical medicine. Whooping cough in an adult. N Engl J Med 2012;366:e39. [Crossref] [PubMed]
- Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA 2010;304:890-6. [Crossref] [PubMed]
- Vaz-de-Lima LR, Martin MD, Pawloski LC, et al. Serodiagnosis as adjunct assay for pertussis infection in Sao Paulo, Brazil. Clin Vaccine Immunol 2014;21:636-40. [Crossref] [PubMed]
- Miyashita N, Kawai Y, Yamaguchi T, et al. Evaluation of serological tests for diagnosis of Bordetella pertussis infection in adolescents and adults. Respirology 2011;16:1189-95. [Crossref] [PubMed]
- Miyashita N, Akaike H, Teranishi H, et al. Diagnostic value of symptoms and laboratory data for pertussis in adolescent and adult patients. BMC Infect Dis 2013;13:129. [Crossref] [PubMed]
- Mertens PL, Stals FS, Steyerberg EW, et al. Sensitivity and specificity of single IgA and IgG antibody concentrations for early diagnosis of pertussis in adults: an evaluation for outbreak management in public health practice. BMC Infect Dis 2007;7:53. [Crossref] [PubMed]
- May ML, Doi SA, King D, et al. Prospective evaluation of an Australian pertussis toxin IgG and IgA enzyme immunoassay. Clin Vaccine Immunol 2012;19:190-7. [Crossref] [PubMed]
- Abu Raya B, Bamberger E, Gershtein R, et al. The laboratory diagnosis of Bordetella pertussis infection: a comparison of semi-nested PCR and real-time PCR with culture. Eur J Clin Microbiol Infect Dis 2012;31:619-22. [Crossref] [PubMed]
- Bergquist SO, Bernander S, Dahnsjo H, et al. Erythromycin in the treatment of pertussis: a study of bacteriologic and clinical effects. Pediatr Infect Dis J 1987;6:458-61. [Crossref] [PubMed]
- Altunaiji S, Kukuruzovic R, Curtis N, et al. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev 2007.CD004404. [PubMed]
- Wang K, Bettiol S, Thompson Matthew J, et al. Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst Rev 2014.CD003257. [PubMed]
- Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008;371:1364-74. [Crossref] [PubMed]
- Pratter MR. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest 2006;129:63S-71S. [Crossref] [PubMed]
- Sanu A, Eccles R. Postnasal drip syndrome. Two hundred years of controversy between UK and USA. Rhinology 2008;46:86-91. [PubMed]
- Zhang Q, Ma Q, Huang Z, et al. A Preliminary Study on etiology of Upper Airway Cough Syndrome. Chin J Respir Crit Care Med 2010;9:458-61.
- Poulose V, Bin Mohd I. Prolonged cough presenting with diagnostic difficulty: a study of aetiological and clinical outcomes. Singapore Med J 2011;52:267-70. [PubMed]
- Benich JJ 3rd, Carek PJ. Evaluation of the patient with chronic cough. Am Fam Physician 2011;84:887-92. [PubMed]
- Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology 2013;18:948-56. [Crossref] [PubMed]
- Ryan NM, Gibson PG. Characterization of laryngeal dysfunction in chronic persistent cough. Laryngoscope 2009;119:640-5. [Crossref] [PubMed]
- Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012;(23):3 p preceding table of contents, 1-8.
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol 2010;126:466-76. [Crossref] [PubMed]
- Wilson AM, O'byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med 2004;116:338-44. [Crossref] [PubMed]
- Rodrigo GJ, Yanez A. The role of antileukotriene therapy in seasonal allergic rhinitis: a systematic review of randomized trials. Ann Allergy Asthma Immunol 2006;96:779-86. [Crossref] [PubMed]
- Zuberbier T, Bachert C, Bousquet PJ, et al. GA2 LEN/EAACI Pocket Guide For Allergen-Specific Immunotherapy For Allergic Rhinitis And Asthma. Allergy 2010;65:1525-30. [Crossref] [PubMed]
- Zhang L, Han D. Allergen-specific subcutaneous immunotherapy for allergic rhinitis. Chinese Journal of Otorhinolaryngology Head And Neck Surgery 2007;42:711-6. [PubMed]
- Sanderson AR, Leid JG, Hunsaker D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope 2006;116:1121-6. [Crossref] [PubMed]
- Sanclement JA, Webster P, Thomas J, et al. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope 2005;115:578-82. [Crossref] [PubMed]
- Piromchai P, Thanaviratananich S, Laopaiboon M. Systemic antibiotics for chronic rhinosinusitis without nasal polyps in adults. Cochrane Database Syst Rev 2011.CD008233. [PubMed]
- Cervin A, Wallwork B. Efficacy and safety of long-term antibiotics (macrolides) for the treatment of chronic rhinosinusitis. Curr Allergy Asthma Rep 2014;14:416. [Crossref] [PubMed]
- Aukema AA, Mulder PG, Fokkens WJ. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgery. J Allergy Clin Immunol 2005;115:1017-23. [Crossref] [PubMed]
- Vaidyanathan S, Barnes M, Williamson P, et al. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Ann Intern Med 2011;154:293-302. [Crossref] [PubMed]
- Rimmer J, Fokkens W, Chong LY, et al. Surgical versus medical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst Rev 2014;12:CD006991. [PubMed]
- Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50:1-12. [Crossref] [PubMed]
- Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa. J Investig Allergol Clin Immunol 2006;16:148-55. [PubMed]
- Mortuaire G, De Gabory L, Francois M, et al. Rebound congestion and rhinitis medicamentosa: nasal decongestants in clinical practice. Critical review of the literature by a medical pan el. Eur Ann Otorhinolaryngol Head Neck Dis 2013;130:137-44. [Crossref] [PubMed]
- Laccourreye O, Werner A, Giroud JP, et al. Benefits, limits and danger of ephedrine and pseudoephedrine as nasal decongestants. Eur Ann Otorhinolaryngol Head Neck Dis 2015;132:31-4. [Crossref] [PubMed]
- Eccles R, Martensson K, Chen SC. Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold. Curr Med Res Opin 2010;26:889-99. [Crossref] [PubMed]
- Ma Q, Zhang Q, Huang Z, et al. Study on correlation between curative effects of chlorpheniramine on upper airway cough syndrome and on chronic rhinitis/sinusitis. Journal of Third Military Medical University 2011;33:89-91.
- Yu L, Wei W, Wang L, et al. Upper-airway cough syndrome with latent eosinophilic bronchitis. Lung 2010;188:71-6. [Crossref] [PubMed]
- Terasaki G, Paauw DS. Evaluation and treatment of chronic cough. Med Clin North Am 2014;98:391-403. [Crossref] [PubMed]
- Rank MA, Kelkar P, Oppenheimer JJ. Taming chronic cough. Ann Allergy Asthma Immunol 2007;98:305-13; quiz 313-4, 348.
- Bolser DC. Older-generation antihistamines and cough due to upper airway cough syndrome (UACS): efficacy and mechanism. Lung 2008;186 Suppl 1:S74-7. [Crossref] [PubMed]
- Hoza J, Salzman R, Starek I, et al. Efficacy and safety of erdosteine in the treatment of chronic rhinosinusitis with nasal polyposis - a pilot study. Rhinology 2013;51:323-7. [Crossref] [PubMed]
- Macchi A, Terranova P, Castelnuovo P. Recurrent acute rhinosinusitis: a single blind clinical study of N-acetylcysteine vs ambroxol associated to corticosteroid therapy. Int J Immunopathol Pharmacol 2012;25:207-17. [Crossref] [PubMed]
- Majima Y, Kurono Y, Hirakawa K, et al. Efficacy of combined treatment with S-carboxymethylcysteine (carbocisteine) and clarithromycin in chronic rhinosinusitis patients without nasal polyp or with small nasal polyp. Auris Nasus Larynx 2012;39:38-47. [Crossref] [PubMed]
- Cingi C, Unlu HH, Songu M, et al. Seawater gel in allergic rhinitis: entrapment effect and mucociliary clearance compared with saline. Ther Adv Respir Dis 2010;4:13-8. [Crossref] [PubMed]
- Friedman M, Hamilton C, Samuelson CG, et al. Dead Sea salt irrigations vs Saline irrigations with nasal steroids for symptomatic treatment of chronic rhinosinusitis: a randomized, prospective double-blind study. Int Forum Allergy Rhinol 2012;2:252-7. [Crossref] [PubMed]
- Kassel JC, King D, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev 2010.CD006821. [PubMed]
- Khianey R, Oppenheimer J. Is nasal saline irrigation all it is cracked up to be? Ann Allergy Asthma Immunol 2012;109:20-8. [Crossref] [PubMed]
- Ural A, Oktemer TK, Kizil Y, et al. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. J Laryngol Otol 2009;123:517-21. [Crossref] [PubMed]
- Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med 1979;300:633-7. [Crossref] [PubMed]
- Li X, Yu L, Wei W, et al. Comparative study of cough variant asthma responsive and unresponsive to bronchodilator treatment. Journal of Tongji University 2011;32:95-100. (Medical Science).
- Irwin RS, French CT, Smyrnios NA, et al. Interpretation of positive results of a methacholine inhalation challenge and 1 week of inhaled bronchodilator use in diagnosing and treating cough-variant asthma. Arch Intern Med 1997;157:1981-7. [Crossref] [PubMed]
- Bao W, Chen Q, Lin Y, et al. Efficacy of procaterol combined with inhaled budesonide for treatment of cough-variant asthma. Respirology 2013;18 Suppl 3:53-61. [Crossref] [PubMed]
- Tagaya E, Kondo M, Kirishi S, et al. Effects of regular treatment with combination of salmeterol/fluticasone propionate and salmeterol alone in cough variant asthma. J Asthma 2015;52:512-8. [Crossref] [PubMed]
- He ZM, Guo YB, Xie CM. Effect of leukotriene receptor antagonist on cough variant asthma. Nan Fang Yi Ke Da Xue Xue Bao 2009;29:694-6. [PubMed]
- Kawai S, Baba K, Matsubara A, et al. The efficacy of montelukast and airway mast cell profiles in patients with cough variant asthma. J Asthma 2008;45:243-50. [Crossref] [PubMed]
- Kita T, Fujimura M, Ogawa H, et al. Antitussive effects of the leukotriene receptor antagonist montelukast in patients with cough variant asthma and atopic cough. Allergol Int 2010;59:185-92. [Crossref] [PubMed]
- Spector SL, Tan RA. Effectiveness of montelukast in the treatment of cough variant asthma. Ann Allergy Asthma Immunol 2004;93:232-6. [Crossref] [PubMed]
- Takemura M, Niimi A, Matsumoto H, et al. Clinical, physiological and anti-inflammatory effect of montelukast in patients with cough variant asthma. Respiration 2012;83:308-15. [Crossref] [PubMed]
- Zhang Y, Miao Q, Cao Y, et al. A multi-centered, randomized-controlled clinical study on suhuang zhike capsule for cough variant asthma. Journal of Traditional Chinese Medicine 2008;49:504-6.
- Fujimura M, Nishizawa Y, Nishitsuji M, et al. Predictors for typical asthma onset from cough variant asthma. J Asthma 2005;42:107-11. [Crossref] [PubMed]
- Matsumoto H, Niimi A, Takemura M, et al. Prognosis of cough variant asthma: a retrospective analysis. J Asthma 2006;43:131-5. [Crossref] [PubMed]
- Nakajima T, Nishimura Y, Nishiuma T, et al. Characteristics of patients with chronic cough who developed classic asthma during the course of cough variant asthma: a longitudinal study. Respiration 2005;72:606-11. [Crossref] [PubMed]
- Brightling CE, Ward R, Woltmann G, et al. Induced sputum inflammatory mediator concentrations in eosinophilic bronchitis And Asthma. Am J Respir Crit Care Med 2000;162:878-82. [Crossref] [PubMed]
- Xie J, Zhang Q, Zhong N, et al. BAL fluid 8-isoprostan e concentrations in eosinophilic bronchitis and asthma. J Asthma 2009;46:712-5. [Crossref] [PubMed]
- Luo W, Lai K, Chen R, et al. Pathological features of airway inflammation in eosinophilic bronchitis. Chinese Journal of Pathophysiology 2006;5:943-7.
- Luo W, Lai K, Chen R, et al. Characteristics of airway inflammatory cells and mediators in eosinophilic bronchitis patients. Zhonghua Jie He He Hu Xi Za Zhi 2005.626-9. [PubMed]
- Barranco P, Fernandez-Nieto M, Del Pozo V, et al. Nonasthmatic eosinophilic bronchitis in a baker caused by fungal alpha-amylase and wheat flour. J Investig Allergol Clin Immunol 2008;18:494-5. [PubMed]
- Di Stefano F, Di Giampaolo L, Verna N, et al. Occupational eosinophilic bronchitis in a foundry worker exposed to isocyanate and a baker exposed to flour. Thorax 2007;62:368-70. [Crossref] [PubMed]
- Krakowiak AM, Dudek W, Ruta U, et al. Occupational eosinophilic bronchitis without asthma due to chloramine exposure. Occup Med (Lond) 2005;55:396-8. [Crossref] [PubMed]
- Pala G, Pignatti P, Moscato G. The use of fractional exhaled nitric oxide in investigation of work-related cough in a hairdresser. Am J Ind Med 2011;54:565-8. [Crossref] [PubMed]
- Pala G, Pignatti P, Perfetti L, et al. Usefulness of basophil activation test in diagnosis of occupational nonasthmatic eosinophilic bronchitis. Allergy 2010;65:927-9. [Crossref] [PubMed]
- Yacoub MR, Malo JL, Labrecque M, et al. Occupational eosinophilic bronchitis. Allergy 2005;60:1542-4. [Crossref] [PubMed]
- Berry MA, Hargadon B, Mckenna S, et al. Observational study of the natural history oFEosinophilic bronchitis. Clin Exp Allergy 2005;35:598-601. [Crossref] [PubMed]
- Brightling CE, Woltmann G, Wardlaw AJ, et al. Development of irreversible airflow obstruction in a patient with eosinophilic bronchitis without asthma. Eur Respir J 1999;14:1228-30. [Crossref] [PubMed]
- Park SW, Lee YM, Jang AS, et al. Development of chronic airway obstruction in patients with eosinophilic bronchitis: a prospective follow-up study. Chest 2004;125:1998-2004. [Crossref] [PubMed]
- Liu CL, Lai KF, Chen RC, et al. The role of airway neurogenic inflammation in gastro-esophageal reflux induced cough. Zhonghua Jie He He Hu Xi Za Zhi 2005;28:520-4. [PubMed]
- Stein MR. Possible mechanisms of influence of esophageal acid on airway hyperresponsiveness. Am J Med 2003;115 Suppl 3A:55S-59S. [Crossref] [PubMed]
- Liu C, Chen R, Luo W, et al. Neurogenic airway inflammation induced by repeated intra-esophageal instillation of HCl in guinea pigs. Inflammation 2013;36:493-500. [Crossref] [PubMed]
- Kollarik M, Brozmanova M. Cough and gastroesophageal reflux: insights from animal models. Pulm Pharmacol Ther 2009;22:130-4. [Crossref] [PubMed]
- Irwin RS, Zawacki JK, Curley FJ, et al. Chronic cough as the sole presenting manifestation of gastroesophageal reflux. Am Rev Respir Dis 1989;140:1294-300. [Crossref] [PubMed]
- Xu X, Wang L, Liu B, et al. Clinical features of non-acidic gastroesophageal reflux cough. Zhonghua Jie He He Hu Xi Za Zhi 2011;34:855-7.
- Chinese research collaboration of gastroesophageal reflux disease. The value of reflux diagnostic questionnaires in the diagnosis of gastroesophageal reflux disease. Chinese Journal of Digestion 2003;23:651-4.
- Chen Q, Xu X, Yu L, et al. Optimal cut-off point of symptom association probability in the diagnosis of gastroesophageal reflux-induced chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2013;36:746-50. [PubMed]
- Yang Z, Xu X, Chen Q, et al. The diagnostic value of symptom index in gastroesophageal reflux-induced chronic cough. Chinese Journal of Internal Medicine 2014;53:108-11. [PubMed]
- Liu B, Yu L, Qiu Z, et al. The diagnostic value of multichannel intraluminal esophageal impedance and pH monitoring in gastroesophageal reflux-related cough. Chinese Journal of Internal Medicine 2012;51:867-70. [PubMed]
- Baldi F, Cavoli C, Ghersi S, et al. Cost-effectiveness of different diagnostic strategies to assess gastro-oesophageal reflux disease in patients with unexplained chronic persistent cough in Italy. Dig Liver Dis 2006;38:452-8. [Crossref] [PubMed]
- Baldi F, Cappiello R, Cavoli C, et al. Proton pump inhibitor treatment of patients with gastroesophageal reflux-related chronic cough: a comparison between two different daily doses of lansoprazole. World J Gastroenterol 2006;12:82-8. [Crossref] [PubMed]
- Kahrilas PJ, Howden CW, Hughes N, et al. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest 2013;143:605-12. [Crossref] [PubMed]
- Ribolsi M, Savarino E, De Bortoli N, et al. Reflux pattern and role of impedance-pH variables in predicting PPI response in patients with suspected GERD-related chronic cough. Aliment Pharmacol Ther 2014;40:966-73. [Crossref] [PubMed]
- Lin S, Xu G, Hu P, et al. Chinese Consensus on Gastroesophageal Reflux Disease. Chinese Journal of Gastroenterology 2007;4:233-9. [PubMed]
- Hiyama T, Yoshihara M, Tanaka S, et al. Effectiveness of prokinetic agents against diseases external to the gastrointestinal tract. J Gastroenterol Hepatol 2009;24:537-46. [Crossref] [PubMed]
- Lv H, Qiu Z. New insight on diagnosis and management of gastric esophageal reflux cough. Zhonghua Jie He He Hu Xi Za Zhi 2014;37:934-6.
- Xu XH, Yang ZM, Chen Q, et al. Therapeutic efficacy of baclofen in refractory gastroesophageal reflux-induced chronic cough. World J Gastroenterol 2013;19:4386-92. [Crossref] [PubMed]
- Sifrim D, Dupont L, Blondeau K, et al. Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005;54:449-54. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-328. [Crossref] [PubMed]
- Xu X, Chen Q, Liang S, et al. A case of non-acidic gastroesophageal reflux cough treated with baclofen. Chinese Journal of General Practitioners 2013;12:230-1.
- Xu X, Chen Q, Liang S, et al. Successful resolution of refractory chronic cough induced by gastroesophageal reflux with treatment of baclofen. Cough 2012;8:8. [Crossref] [PubMed]
- Moraes-Filho JP, Navarro-Rodriguez T, Barbuti R, et al. Guidelines for the diagnosis and management of gastroesophageal reflux disease: an evidence-based consensus. Arq Gastroenterol 2010;47:99-115. [Crossref] [PubMed]
- Bajbouj M, Becker V, Phillip V, et al. High-dose esomeprazole for treatment of symptomatic refractory gastroesophageal reflux disease--a prospective pH-metry/impedance-controlled study. Digestion 2009;80:112-8. [Crossref] [PubMed]
- Becker V, Bajbouj M, Waller K, et al. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther 2007;26:1355-60. [Crossref] [PubMed]
- Fass R, Sontag SJ, Traxler B, et al. Treatment of patients with persistent heartburn symptoms: a double-blind, randomized trial. Clin Gastroenterol Hepatol 2006;4:50-6. [Crossref] [PubMed]
- Shaheen NJ, Crockett SD, Bright SD, et al. Randomised clinical trial: high-dose acid suppression for chronic cough – a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011;33:225-34. [Crossref] [PubMed]
- Jones R, Patrikios T. The effectiveness of esomeprazole 40 mg in patients with persistent symptoms of gastro-oesophageal reflux disease following treatment with a full dose proton pump inhibitor. Int J Clin Pract 2008;62:1844-50. [Crossref] [PubMed]
- Rackoff A, Agrawal A, Hila A, et al. Histamine-receptor antagonists at night improve gastroesophageal reflux disease symptoms for patients on proton pump inhibitor therapy. Dis Esophagus 2005;18:370-3. [Crossref] [PubMed]
- Allen CJ, Anvari M. Does laparoscopic fundoplication provide long-term control of gastroesophageal reflux related cough? Surg Endosc 2004;18:633-7. [Crossref] [PubMed]
- Chandra KM, Harding SM. Therapy Insight: treatment of gastroesophageal reflux in adults with chronic cough. Nat Clin Pract Gastroenterol Hepatol 2007;4:604-13. [Crossref] [PubMed]
- Jeansonne LO 4th, White BC, Nguyen V, et al. Endoluminal full-thickness plication and radiofrequency treatments for GERD: an outcomes comparison. Arch Surg 2009;144:19-24; discussion 24. [Crossref] [PubMed]
- Liang WT, Wu JM, Hu ZW, et al. Laparoscopic Nissen fundoplication is more effective in treating patients with GERD-related chronic cough than Stretta radiofrequency. Minerva Chir 2014;69:121-7. [PubMed]
- Mainie I, Tutuian R, Agrawal A, et al. Fundoplication eliminates chronic cough due to non-acid reflux identified by impedance pH monitoring. Thorax 2005;60:521-3. [Crossref] [PubMed]
- Meneghetti AT, Tedesco P, Galvani C, et al. Outcomes after laparoscopic Nissen fundoplication are not influenced by the pattern of reflux. Dis Esophagus 2008;21:165-9. [Crossref] [PubMed]
- Ranson ME, Danielson A, Maxwell JG, et al. Prospective study of laparoscopic nissen fundoplication in a community hospital and its effect on typical, atypical, and nonspecific gastrointestinal symptoms. JSLS 2007;11:66-71. [PubMed]
- Toomey P, Teta A, Patel K, et al. Transoral incisionless fundoplication: is it as safe and efficacious as a Nissen or Toupet fundoplication? Am Surg 2014;80:860-7. [PubMed]
- Tutuian R, Mainie I, Agrawal A, et al. Nonacid reflux in patients with chronic cough on acid-suppressive therapy. Chest 2006;130:386-91. [Crossref] [PubMed]
- Worrell SG, Demeester SR, Greene CL, et al. Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg Endosc 2013;27:4113-8. [Crossref] [PubMed]
- Zhang C, Wang ZG, Wu JM, et al. A preliminary investigation of laparoscopic fundoplication treatment on gastroesophageal reflux disease-related respiratory symptoms. Surg Laparosc Endosc Percutan Tech 2012;22:406-9. [Crossref] [PubMed]
- Ziora D, Jarosz W, Dzielicki J, et al. Citric acid cough threshold in patients with gastroesophageal reflux disease rises after laparoscopic fundoplication. Chest 2005;128:2458-64. [Crossref] [PubMed]
- Ogawa H, Fujimura M, Takeuchi Y, et al. Efficacy of itraconazole in the treatment of patients with chronic cough whose sputa yield basidiomycetous fungi—Fungus-associated chronic cough (FACC). J Asthma 2009;46:407-12. [Crossref] [PubMed]
- Lindberg A, Sawalha S, Hedman L, et al. Subjects with COPD and productive cough have an increased risk for exacerbations and death. Respir Med 2015;109:88-95. [Crossref] [PubMed]
- Putcha N, Drummond MB, Connett JE, et al. Chronic productive cough is associated with death in smokers with early COPD. COPD 2014;11:451-8. [Crossref] [PubMed]
- Grossman RF, Ambrusz ME, Fisher AC, et al. Levofloxacin 750 mg QD for five days versus amoxicillin/clavulanate 875 mg/125 mg BID for ten days for treatment of acute bacterial exacerbation of chronic bronchitis: a post hoc analysis of data from severely ill patients. Clin Ther 2006;28:1175-80. [Crossref] [PubMed]
- Hui DS, Ip M, Ling T, et al. A multicentre surveillance study on the characteristics, bacterial aetiologies and in vitro antibiotic susceptibilities in patients with acute exacerbations of chronic bronchitis. Respirology 2011;16:532-9. [Crossref] [PubMed]
- Schaberg T, Ballin I, Huchon G, et al. A multinational, multicentre, non-blinded, randomized study of moxifloxacin oral tablets compared with co-amoxiclav oral tablets in the treatment of acute exacerbation of chronic bronchitis. J Int Med Res 2001;29:314-28. [Crossref] [PubMed]
- Starakis I, Gogos CA, Bassaris H. Five-day moxifloxacin therapy compared with 7-day co-amoxiclav therapy for the treatment of acute exacerbation of chronic bronchitis. Int J Antimicrob Agents 2004;23:129-37. [Crossref] [PubMed]
- Swanson RN, Lainez-Ventosilla A, De Salvo MC, et al. Once-daily azithromycin for 3 days compared with clarithromycin for 10 days for acute exacerbation of chronic bronchitis: a multicenter, double-blind, randomized study. Treat Respir Med 2005;4:31-9. [Crossref] [PubMed]
- Goyal V, Chang AB. Combination inhaled corticosteroids and long-acting beta2-agonists for children and adults with bronchiectasis. Cochrane Database Syst Rev 2014;6:CD010327. [PubMed]
- Martínez-García MÁ, Soler-Cataluna JJ, Catalan-Serra P, et al. Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest 2012;141:461-8. [Crossref] [PubMed]
- Tsang KW, Tan KC, Ho PL, et al. Inhaled fluticasone in bronchiectasis: a 12 month study. Thorax 2005;60:239-43. [Crossref] [PubMed]
- Guran T, Ersu R, Karadag B, et al. Withdrawal of inhaled steroids in children with non-cystic fibrosis bronchiectasis. J Clin Pharm Ther 2008;33:603-11. [Crossref] [PubMed]
- Hernando R, Drobnic ME, Cruz MJ, et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. Int J Clin Pharm 2012;34:644-50. [Crossref] [PubMed]
- Kapur N, Bell S, Kolbe J, et al. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev 2009.CD000996. [PubMed]
- Martínez-García MA, Perpina-Tordera M, Roman-Sanchez P, et al. Inhaled steroids improve quality of life in patients with steady-state bronchiectasis. Respir Med 2006;100:1623-32. [Crossref] [PubMed]
- O’Neill B, Bradley JM, Macmahon J, et al. Subjective benefit of inhaled therapies in patients with bronchiectasis: a questionnaire study. Int J Clin Pract 2004;58:441-3. [Crossref] [PubMed]
- Rosen MJ. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest 2006;129:122S-131S. [Crossref] [PubMed]
- Sidhu MK, Mandal P, Hill AT. Bronchiectasis: an update on current pharmacotherapy and future perspectives. Expert Opin Pharmacother 2014;15:505-25. [Crossref] [PubMed]
- Mostafapour E, Mousavi SA, Shahmiri SS, et al. Effects of combination of fluticasone propionate and salmeterol xinafoate on lung function improvement in patients with bronchiectasis. Lijec Vjesn 2009;131 Suppl 6:8-11. [PubMed]
- Chalmers JD, Smith MP, Mchugh BJ, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012;186:657-65. [Crossref] [PubMed]
- White L, Mirrani G, Grover M, et al. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med 2012;106:356-60. [Crossref] [PubMed]
- Altenburg J, De Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251-9. [Crossref] [PubMed]
- Gao YH, Guan WJ, Xu G, et al. Macrolide therapy in adults and children with non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. PloS One 2014;9:e90047. [Crossref] [PubMed]
- Serisier DJ, Martin ML, Mcguckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309:1260-7. [Crossref] [PubMed]
- Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:660-7. [Crossref] [PubMed]
- Yalçin E, Kiper N, Ozcelik U, et al. Effects of clarithromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. J Clin Pharm Ther 2006;31:49-55. [Crossref] [PubMed]
- Wilkinson M, Sugumar K, Milan SJ, et al. Mucolytics for bronchiectasis. Cochrane Database Syst Rev 2014.CD001289. [PubMed]
- Mandal P, Chalmer SJ, Sidhu M, et al. A randomised controlled trial of atorvastatin as a stable treatment in bronchiectasis. Thorax 2014;69:A10-A11. [Crossref] [PubMed]
- Mandal P, Chalmers JD, Graham C, et al. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir Med 2014;2:455-63. [Crossref] [PubMed]
- Daviskas E, Anderson SD, Eberl S, et al. Effect of increasing doses of mannitol on mucus clearance in patients with bronchiectasis. Eur Respir J 2008;31:765-72. [Crossref] [PubMed]
- Daviskas E, Anderson SD, Gomes K, et al. Inhaled mannitol for the treatment of mucociliary dysfunction in patients with bronchiectasis: effect on lung function, health status and sputum. Respirology 2005;10:46-56. [Crossref] [PubMed]
- Daviskas E, Anderson SD, Young IH. Effect of mannitol and repetitive coughing on the sputum properties in bronchiectasis. Respir Med 2010;104:371-7. [Crossref] [PubMed]
- Hart A, Sugumar K, Milan SJ, et al. Inhaled hyperosmolar agents for bronchiectasis. Cochrane Database Syst Rev 2014.CD002996. [PubMed]
- Gopi PG, Subramani R, Narayanan PR. Evaluation of different types of chest symptoms for diagnosing pulmonary tuberculosis cases in community surveys. Indian J Tuberc 2008;55:116-21. [PubMed]
- Otero L, Ugaz R, Dieltiens G, et al. Duration of cough, TB suspects' characteristics and service factors determine the yield of smear microscopy. Trop Med Int Health 2010;15:1475-80. [Crossref] [PubMed]
- Sekandi JN, Neuhauser D, Smyth K, et al. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis 2009;13:508-13. [PubMed]
- Huan GH, Yao J, Lan X. An analysis of 177 endobronchial tuberculosis cases. Chinese Journal of Misdiagnostics 2007;7:5844-5.
- Zhang W. Endobronchial Tuberculosis and chronic Cough. Chinese Community Physician 2006;(20):11.
- World Health Organization. Treatment of tuberculosis: guidelines. World Health Organization, 2010.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992;117:234-42. [Crossref] [PubMed]
- Sebastian JL, Mckinney WP, Kaufman J, et al. Angiotensin-converting enzyme inhibitors and cough. Prevalence in an outpatient medical clinic population. Chest 1991;99:36-9. [Crossref] [PubMed]
- Simon SR, Black HR, Moser M, et al. Cough and ACE inhibitors. Arch Intern Med 1992;152:1698-700. [Crossref] [PubMed]
- Smyrnios NA, Irwin RS, Curley FJ, et al. From a prospective study of chronic cough: diagnostic and therapeutic aspects in older adults. Arch Intern Med 1998;158:1222-8. [Crossref] [PubMed]
- Wang ZH, Lin JT, Li Y, et al. Etiological Diagnosis and Specific Treatment of Chronic Cough in 106 Patients. Acta Academiae Medicinae Sinicae 2007;29:665-8. [PubMed]
- Yu L, Wei W, Lv H, et al. Changes in the spectrum and frequency of causes for chronic cough: a retrospective analysis. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:414-7. [PubMed]
- Morimoto T, Gandhi TK, Fiskio JM, et al. Development and validation of a clinical prediction rule for angiotensin-converting enzyme inhibitor-induced cough. J Gen Intern Med 2004;19:684-91. [Crossref] [PubMed]
- Tseng DS, Kwong J, Rezvani F, et al. Angiotensin-converting enzyme-related cough among Chinese-Americans. Am J Med 2010;123:183.e11-5. [Crossref] [PubMed]
- Salami AK, Katibi IA. Angiotensin converting enzyme-inhibitors associated cough: A prospective evaluation in hypertensives. Annals of African Medicine 2005;4:118-21.
- Leslie SJ, Faulds SA, Rankin A, et al. A randomised controlled study of ramipril dose-escalation packs in clinical practice. Br J Cardiol 2005;12:136-8.
- Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:169S-173S. [Crossref] [PubMed]
- Verma SK, Mishra AK, Jaiswal AK. Leflunomide-induced chronic cough in a rheumatoid arthritis patient with pulmonary tuberculosis. BMJ Case Rep 2013;2013.
- Reiche I, Troger U, Martens-Lobenhoffer J, et al. Omeprazole-induced cough in a patient with gastroesophageal reflux disease. Eur J Gastroenterol Hepatol 2010;22:880-2. [Crossref] [PubMed]
- Psaila M, Fsadni P, Montefort S. Chronic cough as a complication of treatment with statins: a case report. Ther Adv Respir Dis 2012;6:243-6. [Crossref] [PubMed]
- Gadaleta C, Mattioli V, Colucci G, et al. Radiofrequency ablation of 40 lung neoplasms: preliminary results. AJR Am J Roentgenol 2004;183:361-8. [Crossref] [PubMed]
- Harle AS, Blackhall FH, Smith JA, et al. Understanding cough and its management in lung cancer. Curr Opin Support Palliat Care 2012;6:153-62. [Crossref] [PubMed]
- Molassiotis A, Lowe M, Elli SJ, et al. The experience of cough in patients diagnosed with lung cancer. Support Care Cancer 2011;19:1997-2004. [Crossref] [PubMed]
- Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2006;24:3831-7. [Crossref] [PubMed]
- Escobar-Sacristán JA, Granda-Orive JI, Gutierrez Jimenez T, et al. Endobronchial brachytherapy in the treatment of malignant lung tumours. Eur Respir J 2004;24:348-52. [Crossref] [PubMed]
- Molassiotis A, Bailey C, Caress A, et al. Interventions for cough in cancer. Cochrane Database Syst Rev 2010.CD007881. [PubMed]
- Miyamoto H, Sakao Y, Sakuraba M, et al. Usefulness of suplatast tosilate for chronic cough following lung cancer surgery. Gen Thorac Cardiovasc Surg 2009;57:463-6. [Crossref] [PubMed]
- Vertigan AE, Murad MH, Pringsheim T, et al. Somatic Cough Syndrome (Previously Referred to as Psychogenic Cough) and Tic Cough (Previously Referred to as Habit Cough) in Adults and Children: CHEST Guideline and Expert Panel Report. Chest 2015;148:24-31. [Crossref] [PubMed]
- Van den Bergh O, Van Diest I, Dupont L, et al. On the psychology of cough. Lung 2012;190:55-61. [Crossref] [PubMed]
- Anbar RD, Hall HR. Childhood habit cough treated with self-hypnosis. J Pediatr 2004;144:213-7. [Crossref] [PubMed]
- Faught J, Fitzgerald DA. Habit cough and effective therapy. J Paediatr Child Health 2004;40:399-400. [Crossref] [PubMed]
- Fitzgerald DA, Kozlowska K. Habit cough: assessment and management. Paediatr Respir Rev 2006;7:21-5. [Crossref] [PubMed]
- Haydour Q, Alahdab F, Farah M, et al. Management and diagnosis of psychogenic cough, habit cough, and tic cough: a systematic review. Chest 2014;146:355-72. [Crossref] [PubMed]
- Weinberger M. The habit cough syndrome and its variations. Lung 2012;190:45-53. [Crossref] [PubMed]
- Sun C, Bin L. Back cover a case report of cardiogenic cough. Chinese Journal of Contemporary Pediatrics 2008;10:F0004.
- Chang C, Chenchao S, Lei D, et al. Pleomorphic adenoma of the subglottis mistreated as chronic obstructive pulmonary disease, report of a case. Ann Thorac Cardiovasc Surg 2011;17:283-6. [Crossref] [PubMed]
- Zapparoli M, Trolese AR, Remo A, et al. Subglotic malt-lymphoma of the larynx: an unusual presentation of chronic cough. Int J Immunopathol Pharmacol 2014;27:461-5. [Crossref] [PubMed]
- Yi F, Luo W, Chen Q, et al. One laryngeal carcinoma patient with chronic cough as the only symptom. Chinese Journal of lung Disease (Electronic edition) 2014;5:90-1.
- Parakh A, Singh V. Hypoplastic epiglottis in a nonsyndromic child: a rare cause of chronic cough. J Craniofac Surg 2013;24:e477-9. [Crossref] [PubMed]
- Tan Y, Lin L, Lai K, et al. Delayed diagnosis of a chronic cough patient caused by ectopic salivary gland of the tongue. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:473-4.
- Birring SS, Passant C, Patel RB, et al. Chronic tonsillar enlargement and cough: preliminary evidence of a novel and treatable cause of chronic cough. Eur Respir J 2004;23:199-201. [Crossref] [PubMed]
- Gurgel RK, Brookes JT, Weinberger MM, et al. Chronic cough and tonsillar hypertrophy: a case series. Pediatr Pulmonol 2008;43:1147-9. [Crossref] [PubMed]
- Pai V, Thomas H, Stewart C. Long uvula: an unusual cause of chronic cough. Postgrad Med J 2004;80:116. [Crossref] [PubMed]
- Faruqi S, Fahim A, Morice AH. Chronic cough and obstructive sleep apnoea: reflux-associateD cough hypersensitivity? Eur Respir J 2012;40:1049-50. [Crossref] [PubMed]
- Simsek PO, Ozcelik U, Demirkazik F, et al. Tracheobronchopathia osteochondroplastica in a 9-year-old girl. Pediatr Pulmonol 2006;41:95-7. [Crossref] [PubMed]
- Chien YC, Wang HC, Chang YC, et al. Uncommon chronic cough caused by tracheobronchopathia osteochondroplastica. Thorax 2012;67:1021-2. [Crossref] [PubMed]
- Maimon N, Marras T, Hwang D, et al. A 46-year-old female with dyspnoea, stridor and chronic cough. Eur Respir J 2006;28:666-9. [Crossref] [PubMed]
- El-Kersh K, Perez RL, Gauhar U. A. 63-year-old man with a chronic cough and an endobronchial lesion. Diagnosis: Endobronchial hamartoma. Chest 2014;145:919-22. [Crossref] [PubMed]
- Danielson GP, Jedlovszky V, Landrigan GP. Tracheal diverticulum: a rare finding in a patient with worsening chronic cough. Ear Nose Throat J 2008;87:474-5. [PubMed]
- Ip JJ, Hui PK, Lam SH, et al. Mounier-Kuhn syndrome: an unusual underlying cause for chronic coughs and recurrent pneumonias. Hong Kong Med J 2013;19:365 e363-64.
- Lou Y. The analysis of etiology, diagnosis and treatment of chronic cough in children. China Practical Medical 2011;12:111-2.
- Huankang Z, Kuanlin X, Xiaolin H, et al. Comparison between tracheal foreign body and bronchial foreign body: a review of 1,007 cases. Int J Pediatr Otorhinolaryngol 2012;76:1719-25. [Crossref] [PubMed]
- Ogawa H, Fujimura M, Takeuchi Y, et al. Chronic cough management: dealing with a sensation of mucus in the throat. Respirology 2013;18:732-3. [Crossref] [PubMed]
- Mariotta S, Ricci A, Papale M, et al. Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:173-81. [PubMed]
- Ganesan N, Ambroise MM, Ramdas A, et al. Pulmonary alveolar microlithiasis: an interesting case report with systematic review of Indian literature. Front Med 2015;9:229-38. [Crossref] [PubMed]
- Ogawa H, Fujimura M, Takeuchi Y, et al. The importance of basidiomycetous fungi cultured from the sputum of chronic idiopathic cough: a study to determine the existence of recognizable clinical patterns to distinguish CIC from non-CIC. Respir Med 2009;103:1492-7. [Crossref] [PubMed]
- Behr J. The diagnosis and treatment of idiopathic pulmonary fibrosis. Dtsch Arztebl Int 2013;110:875-81. [PubMed]
- Cordeiro CR, Alfaro TM, Freitas S. Clinical case: differential diagnosis of idiopathic pulmonary fibrosis. BMC Res Notes 2013;6 Suppl 1:S1. [Crossref] [PubMed]
- Moline J, Ngeow J, Rajiah P, et al. Evil lurks in the heart of man: cardiac paraganglioma presenting as recurrent dyspnoea and chronic cough. BMJ Case Rep 2011;2011.
- Moschos C, Kalomenidis I, Roussos C, et al. A 35-year-old male with chronic cough. Eur Respir J 2007;29:608-11. [Crossref] [PubMed]
- Agrawaal KK, Dhakal SS, Bhatta N, et al. Chronic cough in thymoma. JNMA J Nepal Med Assoc 2010;49:164-6. [Crossref] [PubMed]
- Ghaffari S, Parvizi R, Pourafkari L. Delayed presentation of posttraumatic aortic false an eurysm with chronic cough. Am J Med Sci 2009;338:525-6. [Crossref] [PubMed]
- Hasdemir C, Musayev O, Kehribar DY, et al. Chronic cough and tachycardia-induced cardiomyopathy in a patient with idiopathic frequent, monomorphic premature ventricular contractions. Pacing Clin Electrophysiol 2013;36:e156-58. [Crossref] [PubMed]
- Niimi A, Kihara Y, Sumita Y, et al. Cough reflex by ventricular premature contractions. Int Heart J 2005;46:923-6. [Crossref] [PubMed]
- Stec S, Dabrowska M, Zaborska B, et al. Premature ventricular complex-induced chronic cough and cough syncope. Eur Respir J 2007;30:391-4. [Crossref] [PubMed]
- Stec SM, Grabczak EM, Bielicki P, et al. Diagnosis and management of premature ventricular complexes-associated chronic cough. Chest 2009;135:1535-41. [Crossref] [PubMed]
- Zeng Y, Xie Z, Wu J, et al. A case of cervical spondylosis characterized by chronic cough. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:471-2.
- Karatayli-Ozgursoy S, Dominik J, Eidelman B, et al. Chronic cough as the presenting symptom of hydrocephalus. South Med J 2010;103:574-7. [Crossref] [PubMed]
- Chambers KJ, Setlur J, Hartnick CJ. Chiari type I malformation: presenting as chronic cough in older children. Laryngoscope 2013;123:2888-91. [Crossref] [PubMed]
- Shellenberger MJ, Smith R, Varma C, et al. Hepatectomy cures a cough: giant cavernous hemangioma in a patient with persistent cough. J Am Osteopath Assoc 2010;110:675-7. [PubMed]
- Fahim A, Faruqi S, Stafford ND, et al. Glomus vagale presenting as chronic cough. Eur Respir J 2010;35:446-7. [Crossref] [PubMed]
- Hayat S, Pitchaikani PK, Williams N, et al. Coeliac disease presenting as chronic cough in an 8-year-old child. BMJ Case Rep 2011;2011.
- Grossman A, Olonovski D, Barenboim E. Hypothyroidism caused by a nonvisible lingual thyroid. Head Neck 2004;26:995-8. [Crossref] [PubMed]
- Sevinç S, Unsal S, Ozturk T, et al. Thoracic endometriosis syndrome with bloody pleural effusion in a 28 year old woman. J Pak Med Assoc 2013;63:114-6. [PubMed]
- Lai K, Fang Z, Yao H. Cough hypersensitivity syndrome: a new concept for chronic idiopathic cough. Medical Journal of Chinese People’s Liberation Army 2014;5:343-9.
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [Crossref] [PubMed]
- Cohen SM, Misono S. Use of specific neuromodulators in the treatment of chronic, idiopathic cough: a systematic review. Otolaryngol Head Neck Surg 2013;148:374-82. [Crossref] [PubMed]
- Chamberlain S, Birring SS, Garrod R. Nonpharmacological interventions for refractory chronic cough patients: systematic review. Lung 2014;192:75-85. [Crossref] [PubMed]
- Chamberlain S, Garrod R, Birring SS. Cough suppression therapy: does it work? Pulm Pharmacol Ther 2013;26:524-7. [Crossref] [PubMed]
- Murry T, Tabaee A, Aviv JE. Respiratory retraining of refractory cough and laryngopharyngeal reflux in patients with paradoxical vocal fold movement disorder. Laryngoscope 2004;114:1341-5. [Crossref] [PubMed]
- Patel AS, Watkin G, Willig B, et al. Improvement in health status following cough-suppression physiotherapy for patients with chronic cough. Chron Respir Dis 2011;8:253-8. [Crossref] [PubMed]
- Kovesi T, Rubin S. Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest 2004;126:915-25. [Crossref] [PubMed]
- Legrand C, Michaud L, Salleron J, et al. Long-term outcome of children with oesophageal atresia type III. Arch Dis Child 2012;97:808-11. [Crossref] [PubMed]
- Redding GJ. Bronchiectasis in children. Pediatr Clin North Am 2009;56:157-71. xi. [Crossref] [PubMed]
- Rosenfeld M, Davis S, Brumback L, et al. Inhaled hypertonic saline in infants and toddlers with cystic fibrosis: short-term tolerability, adherence, and safety. Pediatr Pulmonol 2011;46:666-71. [Crossref] [PubMed]
- Simpson T, Ivey J. Toddler with a chronic cough. Pediatr Nurs 2005;31:48-9. [PubMed]
- Stannard W, Rutman A, Wallis C, et al. Central microtubular agenesis causing primary ciliary dyskinesia. Am J Respir Crit Care Med 2004;169:634-7. [Crossref] [PubMed]
- Tireli GA, Ozbey H, Temiz A, et al. Bronchogenic cysts: a rare congenital cystic malformation of the lung. Surg Today 2004;34:573-6. [Crossref] [PubMed]
- Lu G, Jin R, Su S, et al. Value of fiberoptic Bronchoscopy in Diagnosis and Therapy of Chronic Cough in Children. Journal of Applied Clinical Pediatrics 1748;2011:1744-1745.
- Wang L, Xia W, Li L, et al. Diagnosis of tracheobronchomalacia in children with electron-bronchoscopy. Chinese Journal of General Practitioners 2008;7:410-1.
- Chang AB, Berkowitz RG. Cough in the pediatric population. Otolaryngol Clin North Am 2010;43:181-8. xii. [Crossref] [PubMed]
- Kompare M, Weinberger M. Protracted bacterial bronchitis in young children: association with airway malacia. J Pediatr 2012;160:88-92. [Crossref] [PubMed]
- O’Grady KA, Grimwood K, Cripps A, et al. Does a 10-valent pneumococcal-Haemophilus influenzae protein D conjugate vaccine prevent respiratory exacerbations in children with recurrent protracted bacterial bronchitis, chronic suppurative lung disease and bronchiectasis: protocol for a randomised controlled trial. Trials 2013;14:282. [Crossref] [PubMed]
- Philipson K, Goodyear-Smith F, Grant CC, et al. When is acute persistent cough in school-age children and adults whooping cough? A prospective case series study. Br J Gen Pract 2013;63:e573-9. [Crossref] [PubMed]
- Spector SL. Chronic cough: the allergist's perspective. Lung 2008;186 Suppl 1:S41-47. [Crossref] [PubMed]
- Mi R, Fu J, Wang X, et al. Clinical research of Bordetella pertussis infection in infants with Prolonged cough. Zhonghua Yi Xue Za Zhi 2013;93:1721-5. [PubMed]
- Boufersaoui A, Smati L, Benhalla KN, et al. Foreign body aspiration in children: experience from 2624 patients. Int J Pediatr Otorhinolaryngol 2013;77:1683-8. [Crossref] [PubMed]
- Midulla F, Guidi R, Barbato A, et al. Foreign body aspiration in children. Pediatr Int 2005;47:663-8. [Crossref] [PubMed]
- Samarei R. Survey of foreign body aspiration in airways and lungs. Glob J Health Sci 2014;6:130-5. [Crossref] [PubMed]
- Shubha AM, Das K. Tracheobronchial foreign bodies in infants. Int J Pediatr Otorhinolaryngol 2009;73:1385-9. [Crossref] [PubMed]
- Tomaske M, Gerber AC, Stocker S, et al. Tracheobronchial foreign body aspiration in children - diagnostic value of symptoms and signs. Swiss Med Wkly 2006;136:533-8. [PubMed]
- Chummun D, Lu H, Qiu Z. Empiric treatment of chronic cough in adults. Allergy Asthma Proc 2011;32:193-7. [Crossref] [PubMed]
- Gladu RH, Hawkins CA. Combatting the cough that won't quit. J Fam Pract 2012;61:88-93. [PubMed]
- Levine BM. Systematic evaluation and treatment of chronic cough in a community setting. Allergy Asthma Proc 2008;29:336-42. [Crossref] [PubMed]
- Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:222S-231S. [Crossref] [PubMed]
- Niimi A, Ohbayashi H, Sagara H, et al. Cough variant and cough-predominant asthma are major causes of persistent cough: a multicenter study in Japan. J Asthma 2013;50:932-7. [Crossref] [PubMed]
- Zhang Q, Ma Q, Cheng X, et al. The study of diagnosis and treatment of chronic cough based empirical therapy. Chinese Journal of Lung Disease (Electronic edition) 2010;3402-7.
- Gahbauer M, Keane P. Chronic cough: Stepwise application in primary care practice of the ACCP guidelines for diagnosis and management of cough. J Am Acad Nurse Pract 2009;21:409-16. [Crossref] [PubMed]
- Lee J, Kim M, Kim JH, et al. A cheaper, faster way to resolve chronic cough. J Fam Pract 2007;56:641-6. [PubMed]
- Wei W, Yu L, Wang Y, et al. Efficacy and safety of modified sequential three-step empirical therapy for chronic cough. Respirology 2010;15:830-6. [Crossref] [PubMed]
- Yu L, Qiu Z, Lu H, et al. Clinical benefit of sequential three-step empirical therapy in the management of chronic cough. Respirology 2008;13:353-8. [Crossref] [PubMed]
- Yancy WS Jr, Mccrory DC, Coeytaux RR, et al. Efficacy and tolerability of treatments for chronic cough: a systematic review and meta-analysis. Chest 2013;144:1827-38. [Crossref] [PubMed]
- Dicpinigaitis PV, Gayle YE, Solomon G, et al. Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough. Respir Med 2009;103:902-6. [Crossref] [PubMed]
- LaForce C, Gentile DA, Skoner DP. A randomized, double-blind, parallel-group, multicenter, placebo-controlled study of the safety and efficacy of extended-release guaifenesin/pseudoephedrine hydrochloride for symptom relief as an adjunctive therapy to antibiotic treatment of acute respiratory infections. Postgrad Med 2008;120:53-9. [Crossref] [PubMed]
- Yakoot M, Salem A, Omar AM. Clinical efficacy of farcosolvin syrup (ambroxol-theophylline-guaiphenesin mixture) in the treatment of acute exacerbation of chronic bronchitis. Int J Chron Obstruct Pulmon Dis 2010;5:251-6. [Crossref] [PubMed]
- Federspil P, Wulkow R, Zimmermann T. Effects of standardized Myrtol in therapy of acute sinusitis--results of a double-blind, randomized multicenter study compared with placebo. Laryngorhinootologie 1997;76:23-7. [Crossref] [PubMed]
- Meister R, Wittig T, Beuscher N, et al. Efficacy and tolerability of Myrtol standardized in long-term treatment of chronic bronchitis. Arzneimittelforschung 1999;49:351-8. [PubMed]
- Zhou ZY. Internal Medicine of Traditional Chinese Medicine. Beijing: China Press of Chinese Traditional Medicine, 2005.
- Li ZY. Zhang JingYue’s Medical encyclopedia. Beijing: China Press of Chinese Traditional Medicine, 1999.
- Zhu W. National standard of the application of TCM in disease diagnosis and management. Hunan Science and Technology Press, 1999.
- China Association of Chinese Medicine Internal Medicine Branch, Committee on Lung Disease. Expert consensus on traditional Chinese medicine in diagnosis and treatment of cough. Journal of Traditional Chinese Medicine 2011;52:896-9.
- National pharmacopoeia commission. Pharmacopoeia of the People’s Republic of China (Volume I). Beijing: China Pharmaceutical Technology Press, 2010.
- Zhang BL. Internal Medicine of Traditional Chinese Medicine. People’s Medical Publishing House, 2012.
- Jiang M, Liao L, Luo W, et al. Quality Assessment of Clinical Practice Guidelines for Diagnosis and Management of Cough in China. Chin J Evid-Based Med 2015;15:409-13.