
Improving clinical outcomes in sepsis and multiple organ dysfunction through precision medicine
Introduction
Sepsis is an ancient syndrome, as the term “sipo” (‘‘I rot’’ in Greek) was first used in a medical sense in the poems of Homer (1). Two thousand and seven hundred years later, sepsis remains a serious human disease with significant morbidity and mortality. Moreover, sepsis is increasingly common due to an aging population with multiple co-morbid illnesses that are being more aggressively treated with surgery and multiple complex therapies, including biologic and immunosuppressive therapies such as cancer chemotherapy (2-5).
Sepsis occurs in 5–10% of all hospitalized patients (4,6-8), and is the most common cause of mortality in ICUs, being fatal in at least 20–30% of patients affected (3,6,8-11). Global estimates suggest 30 million episodes annually with at least 6 million deaths (2,7,12,13). Sepsis is costly with regards to healthcare resources, as care for sepsis consumes up to 45% of total ICU costs and has become the leading healthcare expense for hospitalized patients (6,10,14,15). Although more patients are surviving sepsis today in high-income countries, many survivors suffer long-term burdens, including new cognitive and physical functional limitations, poor health-related quality of life (HRQoL), and shortened lifespan (16-18). The burden of sepsis is even greater in low and middle-income countries, leading the WHO in 2017 to declare sepsis a Global Health Priority (13).
Evolving definition of sepsis
The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (3) recently re-defined sepsis as a dysregulated host response to infection leading to life-threatening single or multiple organ dysfunction (MOD) (Figure 1), most commonly cardiovascular, pulmonary, renal, and brain dysfunction (3,19,20). Notably, the definition of sepsis has evolved from simple consideration of systemic inflammation resulting from infection to the current concept of infection-triggered MOD (3,19,20). This shift to septic MOD in both diagnostic focus and particularly management is highly clinically relevant. It is precisely the presence, severity and course of individual and MOD that determine the severity of clinical illness in individual patients with sepsis, the nature and intensity of required ICU care, and the prognosis for in hospital mortality as well as long-term functional morbidity and mortality (3,19,20).
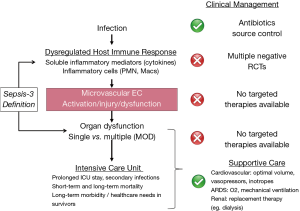
In recent years, clinical outcomes, including survival, have improved in sepsis patients, at least in high-income countries, due in large part to international guidelines established over the past 20 years (5,21-23). These initiatives, such as the “Surviving Sepsis Campaign”, have promoted increased awareness of sepsis, early diagnosis in patients at risk, and protocolized management (5,23). However, clinical outcomes remain poor for many patients with sepsis, especially in those presenting with MOD or developing it during the first few days of ICU stay. Further improvement in short- and long-term clinical outcomes in sepsis will require more specific, effective therapy targeted at the individual patient-specific underlying pathophysiology of sepsis and resulting MOD. The lack of successful development of effective therapies suggests less than full understanding of the pathobiology of sepsis, including of the resulting MOD that largely contributes to morbidity and mortality of sepsis, especially in individual patients with sepsis. This has resulted in the current “imprecise” approach to management of groups or populations of patients, rather than a targeted treatment of individual patients in a “Precision Medicine” approach.
Pathobiology of sepsis
The presence of infection leads to initial activation of the innate immune response. This is driven through recognition of recurring structural patterns, the pathogen-associated molecular patterns (PAMPs), which include microbial components such as lipopolysaccharide (LPS) as well as endogenous host molecules, e.g., heat-shock proteins and DNA fragments (24,25). These PAMPs and tissue injury-related damage-associated molecular patterns (DAMPs) are recognized by 4 families of specific pattern recognition receptors (PRRs), such as toll-like receptors (TLRs). These PRRs are localized on immune and tissue cell surfaces and are critical in mediating the activation of the innate immune response through intracellular signalling cascades. The resulting pro-inflammatory host response is both complex and redundant, involving many soluble inflammatory mediators, including cytokines [e.g., tumor necrosis factor (TNF) α and interleukin (IL) 6] and reactive oxygen/nitrogen species (e.g., nitric oxide (NO) and peroxynitrite), as well as multiple cell types, including neutrophils, macrophages, platelets, and endothelial cells.
Based on this understanding, numerous anti-inflammatory therapies have been proposed and studied, including corticosteroids, anti-cytokine approaches, as well as various other basic research-driven therapies (e.g., activated protein C, β-agonists, and statins). Unfortunately, all of these putative therapies have failed to improve clinical outcomes in human patients with sepsis (25-31).
A potential explanation for the failure of anti-inflammatory therapies is that simultaneous with the septic inflammatory response, there is evidence for an equally complex anti-inflammatory reaction (24,28). This anti-inflammatory response is characterized by activation of anti-inflammatory cytokines (e.g., IL10, IL1 receptor antagonist, TGFβ), and resulting immunosuppression leading to attenuated cellular and humoral responses to pathogens. This immunosuppression is thought to be associated with a higher risk of secondary, opportunistic infections (32,33), although the source/type of secondary infection does not appear to contribute to mortality (34-36). Importantly, this risk of secondary infection is enhanced by other host and treatment factors, including co-morbid illnesses and background immunosuppressive medications, ICU location, and treatment with broad-spectrum antibiotics.
Based on a potential contribution of delayed immunosuppression to late-sepsis mortality, immunostimulatory therapies have also been proposed (28,37). However, this may be of limited benefit, as secondary infections in sepsis patients are common, but not necessarily more so than in non-septic ICU patients, and appear to contribute only modestly to poor sepsis outcomes, including overall mortality (33,34,36).
Unfortunately, not a single novel research-driven approach to sepsis pharmacologic therapy has improved clinical outcomes, and thus none are currently indicated or widely used in the management of sepsis.
MOD
Morbidity and mortality in sepsis are largely due to MOD, especially cardiovascular dysfunction and lung involvement resulting in acute respiratory distress syndrome (ARDS), as well as brain and renal dysfunction (3,6,10,27,38-41). Septic MOD can arise from metastatic infection, but is more commonly driven by the overwhelming host pro-inflammatory response and the resulting cellular and tissue inflammation, injury and dysfunction. Central to the pathophysiology of septic MOD are cardiovascular abnormalities, including involvement and dysfunction of the heart, large blood vessels, and especially the microvasculature (42-44). For example, septic myocardial and macrovascular dysfunction lead to severe hypotension and risk of cardiovascular shock, which, for the most part, are well-addressed clinically using optimal fluid management, inotropes, and vasopressors.
Most importantly, it is septic inflammation, injury and dysfunction of the microvasculature of multiple individual organs that contribute to the risk of septic MOD and the high mortality of sepsis. The homeostatic function of the microvasculature in each organ is fundamentally, locally regulated by microvascular endothelial cells (MVEC). MVEC regulate distribution of blood flow, prevent microvascular neutrophil sequestration and thrombosis, and most importantly, maintain a selective permeability barrier that prevents the leak of proteinaceous fluid and infiltration of neutrophils into tissues and organs. Correspondingly, it is clear that microvascular dysfunction is critically driven by MVEC dysfunction. Septic microvascular/MVEC dysfunction are characterized by vasomotor dysfunction, increased neutrophil adhesion, in situ microvascular thrombosis, and most importantly, impairment of the normal MVEC permeability barrier, leading to extra-vascular tissue leak of high-protein edema and neutrophil transmigration (42-45). Indeed, in 2010 the NHLBI emphasized the importance in sepsis of the “unifying concept of severe endothelial dysfunction syndrome in response to intravascular or extravascular microbial agents that cause multi-organ failure” (46).
This microvascular dysfunction has been identified early in the course of sepsis in humans and is clinically important, being associated with increased mortality (47-50), especially if it persists over time (51). Moreover, there is also evidence specifically for activation, injury and dysfunction of EC in human sepsis, including increased numbers of circulating EC as well as higher plasma levels of markers of EC activation/injury, e.g., intercellular adhesion molecule (ICAM) 1 and vascular endothelial growth factor receptor type 1 (VEGFR1 or FLT1). Importantly, these circulating EC markers correlate with more severe sepsis and higher mortality in patients (52-54).
Septic microvascular and specifically MVEC dysfunction are initiated by the interaction of MVEC with circulating blood components, including neutrophils and soluble inflammatory mediators, such as LPS, TNFα, and IL6. There are several mechanisms of neutrophil-dependent cell and tissue injury including physical interaction (e.g., neutrophil-MVEC adhesion), augmented production of pro-inflammatory cytokines and chemokines as well as reactive oxygen and nitrogen species (e.g., NO, peroxynitrite), release of proteases and peptides (e.g., matrix metalloproteinases), and formation of cytotoxic neutrophil extracellular traps (NETs) (24,25).
Management
Given poor clinical outcomes in sepsis, the standard of care has become early recognition and urgent management through campaigns such as the 2004 “Surviving Sepsis Campaign”, since revised several times (5,21-23). Guidelines encourage use of scoring systems, such as Sequential Organ Failure Assessment (SOFA) (55,56), to “screen” patients with documented or suspected infection in order to detect organ dysfunction in a more timely fashion to establish the diagnosis of sepsis. Once sepsis is identified, clinical management “bundles” based on best evidence and expert consensus are recommended, e.g., early blood cultures, broad spectrum antibiotics, and source control for infection. In addition, aggressive supportive therapy is critical, including adequate initial volume resuscitation and vasopressors to treat hypotension/shock, as well as hemodynamic and/or lactate monitoring to guide further fluid/vasopressor management (5,23,57). Strong evidence supports an association between adherence to bundle management components and improved survival of patients with sepsis (23,58), especially in high-income countries.
However, further sepsis management remains largely limited to supportive measures for some components of MOD, such as supplemental O2 and low-tidal volume mechanical ventilation for ARDS, and renal replacement therapy (e.g., dialysis) for renal dysfunction. It should be noted that there are no available supportive therapies for other septic organ dysfunctions, e.g., acute brain dysfunction. Most disappointing, despite hundreds of clinical trials enrolling tens of thousands of subjects, not a single novel pharmacologic therapy has been “discovered” or approved for the management of sepsis patients (29,30). As a result, there is still no specific, effective therapy available for patients with sepsis, specifically therapies targeted at the injury and/or dysfunction of organs, the microvasculature, or MVEC. It is especially concerning that adherence to recommended protocolized care, such as appropriate early resuscitation, may not improve MVEC activation and/or injury as reflected by plasma markers (59).
The future of sepsis therapy: precision-medicine
Sepsis has always been defined broadly, in order to encompass the greatest number of patients at risk, but this approach has resulted in an obligatory heterogeneous syndrome of various ages, co-morbidities, types of infection, and severity of clinical/physiologic illness. This marked heterogeneity has clearly confounded the development of effective sepsis therapies, as the lack of “significant” overall mean or “average” clinical benefit in trials of sepsis populations could be either failure of the therapy or failure of the diagnostic criteria to identify an appropriately responsive subject (29,30). Optimal clinical management of the individual patient with sepsis is also confounded by this heterogeneity, including the type and severity of the initial infection, the nature, severity, and timing of the dysregulated host immune response, as well as the presence and extent of pathophysiologic microvascular/MVEC dysfunction and resulting organ dysfunction, including the number and specific organs affected. Moreover, further recognized heterogeneity exists around patients’ genetic constitutions, their environments, and their health-related behaviors including their willingness and ability to access timely care. Persistent poor short-term and long-term clinical outcomes in sepsis patients despite “best” current ICU supportive care are almost certainly due to this collective heterogeneity.
This realization has led to many attempts to personalize the management of sepsis patients. The first approach was to identify predictors of worse prognosis in sepsis in order to phenotype and risk-stratify individual patients (Table 1). Based on identified adverse prognostic variables, including demographic and clinical features, as well as physiologic and laboratory parameters, complex clinical/physiologic/laboratory scoring systems were formulated, such as the Acute Physiology and Chronic Health Evaluation (APACHE) (60) that has since been modified several times yielding the current version, APACHE IV (61). The greater focus on MOD in patients with sepsis led to scoring systems that specifically assess the presence and/or severity of organ dysfunction, e.g., SOFA (sequential organ failure assessment) and MOD scores (53,55,62). The routine use of such scoring systems remains controversial, as many are cumbersome and not easily applied clinically, but this remains an area of ongoing development and investigation of simpler scores, e.g., quickSOFA (qSOFA) (63). Relatedly, the PIRO (Predisposition, Insult, Response, Organ Dysfunction) model was an attempt to stratify sepsis patients for clinical trials and for targeted therapies (64,65).
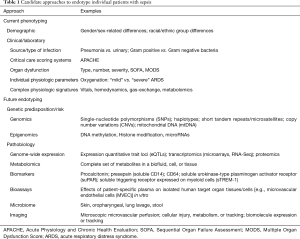
Full table
It is obvious to sepsis researchers and clinicians that the complexity and heterogeneity of sepsis will not permit significant clinical benefits in broad sepsis populations of the reductionist approach of targeting a single effective treatment to any one of the multiple pathobiologic abnormalities identified in pre-clinical and clinical research. International expert panels and guidelines have suggested that future sepsis research must prioritize the understanding of individual patients’ “functional defects” (30,46,66-68). Such an approach may help identify distinct functional sepsis “endotypes” or subgroups of individuals, based on measurable genetic and biologic differences, who are more homogeneous in their pathobiology, prognosis, and response to specific therapy. The very idea of precision arises from phenotyping the pathobiologic features in an individual patient to distinguish them from the “average” sepsis patient (Table 1).
The identification and testing of “biomarkers” represents such an attempt to endotype individual patients, based on the FDA biomarker definition of “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”. Three types of biomarkers could be useful in the clinical management of patients with sepsis, including diagnostic markers, prognostic biomarkers which predict outcomes such as mortality, and predictive biomarkers which predict response to specific therapy.
In humans with sepsis, numerous plasma “biomarkers” [178 by the count of a systematic review in 2010 (69)] have been identified and assessed for their prognostic value (Table 1), including cytokines, cell surface markers or receptors [e.g., cluster designation (CD)14, CD64, TLRs], and markers of EC activation/injury [e.g., VEGFR1/FLT1, angiopoietin (Ang)-2 (59,70,71)]. Unfortunately, no single biomarker or panel of multiple biomarkers has demonstrated validated clinical utility to accurately predict clinical outcomes in individual patients. This is because of multiple issues with the use of biomarkers, including the complex heterogeneity of individual patient-specific immune responses, the lack of correlation between plasma and cellular/tissue molecular processes, and an incomplete understanding of the pathobiology of sepsis, especially of septic MOD (71,72).
Future directions
Significant progress has been made in understanding the pathobiology of sepsis, and in diagnosing and managing human sepsis, specifically ICU care for MOD, which has clearly improved short-term survival. However, available supportive care is costly, and increased sepsis survival comes at the expense of more prolonged ICU and hospital length of stay, more cognitive and physical functional morbidity, lack of return to pre-septic functional status, ongoing rehabilitation and psychosocial resource needs, as well as a higher level of future medical care. Ongoing research will better define the mechanisms of septic MOD, including microvascular injury/dysfunction, septic MVEC pathobiology, and the clinical relevance of basic science observations in animal models and in isolated human cells.
Moreover, multiple-omics technologies and novel “functional” stratification approaches will better endotype individual patients with sepsis and septic MOD, and potentially identify homogeneous groups that are more likely to respond to and benefit from pathobiologically-targeted future therapies in clinical trials as well as in clinical practice (Table 1). Just as importantly, endotyping could also identify individuals who are unlikely to benefit or are more likely to be harmed by specific treatments, encouraging avoidance of such therapies in these individuals, thus avoiding unnecessary harms and enhancing cost-effective resource-allocation.
Endotyping approaches promise great advances in research into both population and individual predisposition to sepsis, MOD, and specific outcomes, but they do not currently offer the necessary analytic speed for bedside utility in clinical decision-making for management of the individual sepsis patient. These important advances will be critical to permit future realization of the exciting potential of a “Precision Medicine” approach to the assessment of the unique genetic and biological characteristics of each individual sepsis patient at the bedside, in order to target therapy to improve short-term and long-term patient-relevant clinical outcomes.
Acknowledgements
Funding: This work was supported by the Heart and Stroke Foundation of Ontario/Canada (G-16-00014621, G-17-0018385), the Canadian Institutes of Health Research (FRN 149038), and the Program of Experimental Medicine of the Department of Medicine, London Health Science Centre.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Funk DJ, Parrillo JE, Kumar A. Sepsis and Septic Shock: A History. Crit Care Clin 2009;25:83-101. [Crossref] [PubMed]
- Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States: A population-based study. Ann Am Thorac Soc 2015;12:216-20. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 2017;318:1241-9. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486-552. [Crossref] [PubMed]
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840-51. [Crossref] [PubMed]
- Navaneelan T, Alam S, Peters PA, et al. Deaths involving sepsis in Canada. Health at a Glance. Statistics Canada catalogue no. 82-624-x.
- Kadri SS, Rhee C, Strich JR, et al. Estimating Ten-Year Trends in Septic Shock Incidence and Mortality in United States Academic Medical Centers Using Clinical Data. Chest 2017;151:278-85. [Crossref] [PubMed]
- Ferrer R, Artigas A, Suarez D, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med 2009;180:861-6. [Crossref] [PubMed]
- Husak L, Marcuzzi A, Herring J, et al. National analysis of sepsis hospitalizations and factors contributing to sepsis in-hospital mortality in Canada. Healthc Q 2010;13:35-41. [Crossref] [PubMed]
- Starr ME, Saito H. Sepsis in old age: review of human and animal studies. Aging Dis 2014;5:126-36. [PubMed]
- Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am J Respir Crit Care Med 2016;193:259-72. [Crossref] [PubMed]
- Seventieth World Health Assembly. Improving the prevention, diagnosis and clinical management of sepsis. Report by the Secretariat 2017;315:1-6.
- Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754-61. [Crossref] [PubMed]
- Tiru B, DiNino EK, Orenstein A, et al. The Economic and Humanistic Burden of Severe Sepsis. Pharmacoeconomics 2015;33:925-37. [Crossref] [PubMed]
- Iwashyna TJ, Ely EW, Smith DM, et al. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010;304:1787-94. [Crossref] [PubMed]
- Yende S, Austin S, Rhodes A, et al. Long-term quality of life among survivors of severe sepsis: Analyses of two international trials. Crit Care Med 2016;44:1461-7. [Crossref] [PubMed]
- Prescott HC, Angus DC. Enhancing recovery from sepsis: A review. JAMA 2018;319:62-75. [Crossref] [PubMed]
- Jacob JA. New Sepsis Diagnostic Guidelines Shift Focus to Organ Dysfunction. JAMA 2016;315:739-40. [Crossref] [PubMed]
- Esposito S, De Simone G, Boccia G, et al. Sepsis and septic shock: New definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist 2017;10:204-12. [Crossref] [PubMed]
- Dellinger RP, Carlet JM, Masur H, et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med 2004;30:536-55. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med 2018;46:997-1000. [Crossref] [PubMed]
- Lewis AJ, Billiar TR, Rosengart MR. Biology and Metabolism of Sepsis: Innate Immunity, Bioenergetics, and Autophagy. Surg Infect (Larchmt) 2016;17:286-93. [Crossref] [PubMed]
- Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016;353:i1585. (Clinical Res ed). [Crossref] [PubMed]
- Moran JL, Graham PL, Rockliff S, et al. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Crit Care 2010;14:R134. [Crossref] [PubMed]
- Sandrock CE, Albertson TE. Controversies in the treatment of sepsis. Semin Respir Crit Care Med 2010;31:66-78. [Crossref] [PubMed]
- Leentjens J, Kox M, Van Der Hoeven JG, et al. Immunotherapy for the adjunctive treatment of sepsis: From immunosuppression to immunostimulation time for a paradigm change? Am J Respir Crit Care Med 2013;187:1287-93. [Crossref] [PubMed]
- Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med 2014;20:195-203. [Crossref] [PubMed]
- Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: A roadmap for future research. Lancet Infect Dis 2015;15:581-614. [Crossref] [PubMed]
- Brown KA, Brown GA, Lewis SM, et al. Targeting cytokines as a treatment for patients with sepsis: A lost cause or a strategy still worthy of pursuit? Int Immunopharmacol 2016;36:291-9. [Crossref] [PubMed]
- Otto GP, Sossdorf M, Claus RA, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 2011;15:R183. [Crossref] [PubMed]
- van Vught LA, Klouwenberg PM, Spitoni C, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA 2016;315:1469-79. [Crossref] [PubMed]
- Zahar JR, Timsit JF, Garrouste-Orgeas M, et al. Outcomes in severe sepsis and patients with septic shock: Pathogen species and infection sites are not associated with mortality. Crit Care Med 2011;39:1886-95. [Crossref] [PubMed]
- Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 2014;40:463-75. [Crossref] [PubMed]
- Angus DC, Opal S. Immunosuppression and secondary infection in sepsis: Part, not all, of the story. JAMA 2016;315:1457-9. [Crossref] [PubMed]
- van der Poll T, van de Veerdonk FL, Scicluna BP, et al. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 2017;17:407-20. [Crossref] [PubMed]
- Bone RC. The sepsis syndrome. Definition and general approach to management. Clin Chest Med 1996;17:175-81. [Crossref] [PubMed]
- Vincent JL, Nelson DR, Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit Care Med 2011;39:1050-5. [Crossref] [PubMed]
- Martin-Loeches I, De Haro C, Dellinger RP, et al. Effectiveness of an inspiratory pressure-limited approach to mechanical ventilation in septic patients. Eur Respir J 2013;41:157-64. [Crossref] [PubMed]
- Guirgis FW, Khadpe JD, Kuntz GM, et al. Persistent organ dysfunction after severe sepsis: A systematic review. J Crit Care 2014;29:320-6. [Crossref] [PubMed]
- Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med 2010;363:689-91. [Crossref] [PubMed]
- Hawiger J, Veach RA, Zienkiewicz J. New paradigms in sepsis: From prevention to protection of failing microcirculation. J Thromb Haemost 2015;13:1743-56. [Crossref] [PubMed]
- Crouser ED, Matthay MA. Endothelial Damage During Septic Shock: Significance and Implications for Future Therapies. Chest 2017;152:1-3. [Crossref] [PubMed]
- Sukriti S, Tauseef M, Yazbeck P, et al. Mechanisms regulating endothelial permeability. Pulm Circ 2014;4:535-51. [Crossref] [PubMed]
- Hawiger JJ, Ginsburg D, Casanova JL, et al. National Heart, Lung, and Blood Institute (NHLBI) Sepsis Meeting. 2010:1-10.
- De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002;166:98-104. [Crossref] [PubMed]
- Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007;49:88-98, 98.e1-2.
- Spanos A, Jhanji S, Vivian-Smith A, et al. Early microvascular changes in sepsis and severe sepsis. Shock 2010;33:387-91. [Crossref] [PubMed]
- De Backer D, Cortes DO, Donadello K, et al. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014;5:73-9. [Crossref] [PubMed]
- Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32:1825-31. [Crossref] [PubMed]
- Mutunga M, Fulton B, Bullock R, et al. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med 2001;163:195-200. [Crossref] [PubMed]
- Shapiro NI, Schuetz P, Yano K, et al. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care 2010;14:R182. [Crossref] [PubMed]
- Skibsted S, Jones AE, Puskarich MA, et al. Biomarkers of endothelial cell activation in early sepsis. Shock 2013;39:427-32. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Ferreira FL. Serial Evaluation of the SOFA Score to Predict Outcome in Critically Ill Patients. JAMA 2001;286:1754. [Crossref] [PubMed]
- Nguyen HB, Jaehne AK, Jayaprakash N, et al. Early goal-directed therapy in severe sepsis and septic shock: Insights and comparisons to ProCESS, ProMISe, and ARISE. Crit Care 2016;20:160. [Crossref] [PubMed]
- Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017;376:2235-44. [Crossref] [PubMed]
- Hou PC, Filbin MR, Wang H, et al. Endothelial Permeability and Hemostasis in Septic Shock: Results From the ProCESS Trial. Chest 2017;152:22-31. [Crossref] [PubMed]
- Knaus WA, Zimmerman J, Wagner D, et al. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med 1981;9:591-7. [Crossref] [PubMed]
- Zimmerman JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit Care Med 2006;34:1297-310. [Crossref] [PubMed]
- Peres Bota D, Melot C, Ferreira FL, et al. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med 2002;28:1619-24. [Crossref] [PubMed]
- Serafim R, Gomes JA, Salluh J, et al. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome Criteria for the Diagnosis of Sepsis and Prediction of Mortality: A Systematic Review and Meta-Analysis. Chest 2018;153:646-55. [Crossref] [PubMed]
- Vincent JL, Wendon J, Groeneveld J, et al. The PIRO concept: O is for organ dysfunction. Crit Care 2003;7:260. [Crossref] [PubMed]
- Rubulotta F, Marshall JC, Ramsay G, et al. Predisposition, insult/infection, response, and organ dysfunction: A new model for staging severe sepsis. Crit Care Med 2009;37:1329-35. [Crossref] [PubMed]
- Davenport EE, Burnham KL, Radhakrishnan J, et al. Genomic landscape of the individual host response and outcomes in sepsis: A prospective cohort study. Lancet Respir Med 2016;4:259-71. [Crossref] [PubMed]
- Scicluna BP, van Vught LA, Zwinderman AH, et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med 2017;5:816-26. [Crossref] [PubMed]
- Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving Sepsis Campaign: Research Priorities for Sepsis and Septic Shock. Crit Care Med 2018;46:1334-56. [Crossref] [PubMed]
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 2010;14:R15. [Crossref] [PubMed]
- Gibot S, Béné MC, Noel R, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med 2012;186:65-71. [Crossref] [PubMed]
- Larsen FF, Petersen JA. Novel biomarkers for sepsis: A narrative review. Eur J Intern Med 2017;45:46-50. [Crossref] [PubMed]
- Biron BM, Ayala A, Lomas-neira JL. Biomarkers for Sepsis: What Is and What Might Be? Biomark Insights 2015;10:7-17. [PubMed]


