Biopsy needles for mediastinal lymph node sampling by endosonography: current knowledge and future perspectives
Introduction
Endoscopic ultrasound [endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and esophageal ultrasound-guided fine needle aspiration (EUS-FNA)] are well established tools in the diagnosis and staging of a variety of diseases, especially lung cancer (1-3). It is possible to sample mediastinal lymph nodes, lung tumors as well as the liver, the left adrenal gland and the subdiaphragmatic lymph nodes (2,3). EUS can be also performed with the small EBUS—endoscope, the so called EUS-B procedure (4).
Considering the mainly cytological nature of fine needle aspirates, as well as the small volume of sampled material, it is increasingly important to yield sufficient high-quality material for comprehensive molecular analysis. A reasoned, patient-tailored and evidence-supported choice of the most adequate needle represents a pivotal step in order to achieve the best diagnostic yield in several thoracic diseases. In this context, a thorough knowledge of the technical aspects in connection with endosonography [EBUS-TBNA and EUS(-B-)FNA] is mandatory to warrant the success of the procedure.
Recent guidelines (5,6) addressed several of these technical aspects, including the choice of sedation (moderate vs.deep), the use or active or passive suction, the importance of the needle size, the number of passes, the use of ROSE (rapid on-site evaluation) and the importance of the needle size.
About the latter the choice of needle gauge (G), it is left to the operator and is usually based on the vascularity of the lesion, on how much the target site is angulated and on the need of histological rather than cytological material. As known, in fact, the gauge represents the unit of thickness of a metal sheet or wire. As the gauge number increases, the thickness drops by 10 percent (7). For such a reason, higher gauge needles (22G to 25G needles) are thinner and typically able to provide only cytological samples, smaller gauges needle (19G to 21G needles) are thicker and often able to warrant a small histologic core sample. More details about needles’ features are given further.
Since both EBUS-TBNA and EUS-B-FNA are gaining ground fast, there is a huge interest in the development of improved needles for the procedures. Consequently, there is a need to get a better overview of these needles, especially in respect to the quality of the biopsies obtained. It is important to emphasize that it is no longer enough to assess the quality of the needles judged solely on their ability to obtain material for a diagnosis defined as the presence of cancer.
Especially the ongoing developments in the treatment of patients with metastatic non-small cell lung cancer (NSCLC) poses new demands to the ability of the needles to obtain biopsies suitable for advanced analyses for example molecular testing of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS 1 proto-oncogene, and new biomarkers such as programmed death ligand 1 (PD-L1). We have very sparse knowledge in respect to these aspects. However, theoretically you may expect larger needles to obtain more material than smaller needles, which in turn should increase the likelihood of being able to perform the necessary analyses. In general, for what about thoracic diseases, it is stated that cytological samples can be used for the evaluation of patients with cancer, both for the diagnosis and subtyping, as well as for the evaluation of patients’ benign conditions like sarcoidosis, whereas histological samples are required for subtyping lymphoproliferative disorders (3).
Needles
General characteristics of an ideal needle
Ideally, the needle for ultrasound guided endoscopic fine needle aspiration should have specific features. Obviously, it should be resistant enough to drill the central airway wall, without the risk of damage or, even worse, breakages associated with the contact with cartilages or during the needle pushing up and down. At the same time, a performing needle should be flexible enough to be inserted into the operating channel of the bronchial endoscope without making the tip of the scope too stiff to be flexed to the proper position for sampling. Furthermore, it should be echogenic in order to warrant the best real time visualization during the puncturing. Finally, it should be shaped and designed with the adequate inner lumen and provide the needed amount of tissue (see Figure 1).
Nitinol, a metal alloy of nickel and titanium, currently represents the material of choice in biomedical needles’ research since it accomplishes with all the above-mentioned characteristics (8). Indeed, also a chrome-cobalt alloy needle has been proposed and is commercially available (AcquireTM, Boston Scientifics) (9). Recently, new conception needles have also been made available (Vizishot2TM, Olympus) (Figure 2). According to preliminary results (10), they are proven to be feasible and safe with a promising diagnostic yield (see further). Moreover, their improved design showed a greater degree of flexion (up to 84), and a higher echogenicity due to the presence of echogenic spiral stripes along the outer needle’s surface.
The EchoTip ProCore needle, is designed to provide core biopsy, in comparison to others with sizes of 21G or 22G, that obtain only cytological specimens, since they have a fissure close to the tip for histological sampling. There are no prospective comparative studies between EchoTip ProCore versus other needles in diagnosis of pulmonary diseases. One prospective study is underway, which compares 22G ProCore needle with a standard 22G needle (11).
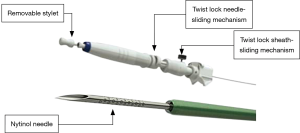
Apart from the needle itself, a conventional EBUS-TBNA device also comprises a twist lock sheath-sliding mechanism to make the control of the needle during the puncture easier, to avoid untoward needle’s movement and a stylet filling the core of the needle that has to be retracted in order to allow a proper sampling (Figure 3). Finally, a suction is applied.
Anecdotal reports of malfunctioning of needles were published, but no safety concerns have never been raised. Release of metal particles due to friction between the stainless-steel needle and nitinol stylet has been reported (12) but no direct consequence for patient’s safety were described. Not proper sheath sliding shaft and needle breakage are other described anomalies (13,14).
No prospective comparative studies showing superiority of one needle to another, currently exist. Future research should focus on clarifying these unsolved issues.
In Table 1, an overview of the different needles available for EBUS-TBNA and EUS-B-FNA is given.

Full table
21G, 22G and 25G needles
Before the introduction of the 19G needles, the needles used in the clinical practice are 21G, 22G and 25G.
In the AQuIRE project (American College of Chest Physicians Quality Improvement Registry, Education and Evaluation), Yarmus et al. (15) found no difference in the sample adequacy or in the diagnostic yield between the 21G and the 22G needle, but the presence of rapid onsite cytological evaluation (ROSE) was associated with significantly fewer needle passes per procedure when using the 21G needle. Nakajima and colleagues (16) evaluated 45 lesions by EBUS-TBNA using both a 21G and a 22G needle. They found no difference in the diagnostic yield between the two needles. However, two patients with adenocarcinoma were only diagnosed with the 21G needle and they reported a better-preserved histological structure of the samples obtained with the 21G needle, but more blood contamination. Moreover, an improvement in preservation of non-necrotizing granulomas was seen with the 22G needle, but it was not statistically significant. A comparison of 21G and 22G needles was also made by Jeyabalan et al. (17). In a cohort of 303 patients, there were no differences in the diagnostic ability for demonstrating malignancy, whereas the 21G needle demonstrated a superior tissue samples characterization for benign conditions especially non-caseating granulomas (sarcoidosis) (18). Histopathological assessment of NSCLC was significantly better with samples obtained with 21G needles compared to 22G needles. A difference in favor of the 21G needle was detected in a study by Saji et al. (18): inadequate material was observed with 22G but not with 21G needle and the overall diagnostic accuracy in cytology, histology, and combined cytology and/or histology was higher with the 21G needle, suggesting that the increased sample volume using a 21G needle (463±71 µm) rather than a 22G needle (460±50 µm) may improve the diagnostic yield (19). Recently, Rotolo et al. (19) in a retrospective multicenter study of selected patients with cancer described their experience with EBUS-TBNA in 485 lung cancer patients suspected for N2 disease, using a 21G or 22G needle. The multivariate analysis may have showed that the 21G needle was associated with a better diagnostic yield. Vaidya et al. (20) investigated the diagnostic yield of a 21G EBUS-TBNA needle for histological and cytological evaluation. In their cohort, compared to EBUS-TBNA cytology, EBUS-TBNA histology was found to have a higher diagnostic yield and negative predicted value (NPV): the sensitivity and the nNPV was 85% and 43% for histology and 65% and 14% for cytology. They concluded also that EBUS-TBNA histology combined with the cytology improved the overall diagnostic yield of EBUS-TBNA; however, it has to be mentioned that in this report the sensitivity of cytology for malignant disease was 78%, significantly lower than in other studies.
The recently produced Vizishot 21G needle provides an angulation of 51° and has a wider outer diameter that provides stiffness for needle penetration, and is designed to provide a larger sample; the Vizishot 22G needle can be bended up to 60° and is designed to offer more flexibility to reach lymph nodes stations that otherwise would be difficult to reach.
The 22G nitinol needle may allow an improved degree of flexibility and prevent needle deformation. Nitinol is a material resistant to bending, which could occur when the operator curves the EBUS scope when taking the biopsies. This characteristic is supposed to result in a straighter and more precise position while taking biopsies. A comparison between the conventional 22G needle and the new Nitinol needle was carried out by Izumo and coworkers (21). The use of the newer needle resulted in a shorter procedure time and in a higher diagnostic yield for histologic specimens. However, these results must be interpreted with great caution, since patients who underwent EBUS-TBNA with the new 22G needle were compared with a historical control group where the traditional 22G needle was used, meaning that other factors, then the type of the needle, may have influenced the results.
Also, 22G and 25G needles have been proposed in order to collect histological biopsy: these needles are 33% more flexible than the conventional 22G and have an adjustable needle extension up to 5 cm (22). So far, it has not been proven that one needle is better than the other, even though several minor advantages have been reported in some studies with the use of 21G needle (5). The only randomized clinical trial was performed by Oki et al. (23) who did not find a superiority of one needle over the other. No differences were found between the two groups of patients in terms of target size, lymph node stations, or prevalence of the disease. There was a trend of more non-representative samples with the 21G needles.
In conclusion, the practical significance of all these findings is unknown. There is no solid basis for a simple recommendation. No clear superiority of one needle over another has been documented so far, but larger needles may seem to perform better especially in non- malignant disorders.
19G needles
The expected angulation of the distal end of the endoscope in some cases may be crucial for the choice of needle. For example, station five and other targets may require angulation and accordingly a more flexible needle could be useful. A flexible 19G flexible needle is available. This needle was designed to obtain high quantities of high-quality specimen, even in challenging locations. Nineteen-gauge needles have been used for a long time during EUS-FNA (endosonography via the esophagus with the use of the large gastroenterological endoscope) with an excellent diagnostic accuracy and with good results in the subtyping of lymphoproliferative disorders (24). Similarly, the recently introduced flexible EBUS 19G needle has demonstrated a promising diagnostic yield, with a great potential in the subtyping of lymphoproliferative disorders and in molecular testing of NSCLC (11).
In comparison to samples collected with a 22G needle (25), samples collected with the 19G needle are larger and may have less blood contamination with a diagnostic yield at least comparable to the 22G needle [mass and volume of the 19G needle sample and of the 22G needle sample: 33.78±47.48 vs. 25.18±32.08 mg (P<0.002) and 11.40±13.91 vs. 6.91±6.42 mm3 (P<0.0004), respectively].
In a cohort of 48 patients that underwent EBUS-TBNA with a 22G needle and subsequently with a 19G needle, the latter allowed a significant increase in the diagnostic yield from 92% to 99%. Four patients (8%) received a diagnosis only with a 19G needle and in 3 cases (6%) the diagnosis was provided only by the 22G needle (26). On the other hand, the 19G needle did not show a superiority over a 22G needle, however, the 19G needle samples had the advantage to be less bloody than 22G needle samples but, conversely, were significantly less frequently adequate (27).
Tremblay et al. reported a diagnostic yield of 77.3% (119/154 cases); 21 patients were found to have a true benign lymphocytosis and 14 samples were not diagnostic or adequate. The overall sample adequacy rate of 90.9%, of 88.6% for solid tumors, of 91% in suspected sarcoidosis/benign lymphadenopathies and of 85.7% in patients suspected for lymphoma. No difference in the adequacy according to sample location was found (28).
Ben et al. (29) conducted a small study in order to determine if a 19G conventional TBNA needle might be useful. Ten patients were examined. They concluded that the needle may be able to reliably acquire histologic specimens. Similar results were achieved by Trisolini et al. (30): a diagnosis was made in the 100% of cases, also from lung lesions (n=3) and pleural nodule (n=1).
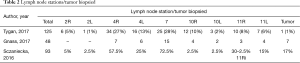
Tygan et al. (10) described the results in 47 selected patients with enlarged hilar and/or mediastinal lymphadenopathy (Table 2) in three centers. The overall diagnostic yield was 89%, with a yield for malignancy of 89% and of 90% for benign disorders. All the samples positive for adenocarcinoma were also adequate for molecular testing and lymphoproliferative disorders were subtyped in 3 out of 4 patients. One case of moderate bleeding occurred, solved with suctioning.
Full table
About sarcoidosis, Balwan et al. reported a diagnostic yield of 93.3%, significantly lower than transbronchial lung biopsy (38%) and endobronchial biopsy (43%) in patients with parenchymal involvement. The latter 2 procedures did not increase the diagnostic yield in any cases (31). Biswas et al. (32) compared the performance of the Excelon 19G needle in patients suspected for sarcoidosis to the performance of the 21G needle. The 19G needle allows a diagnosis of sarcoidosis in 10 cases out of 11, and in one patient non-sarcoid granuloma was found; on the other hand, the 21G needle reached the diagnosis in 2 cases (out of 11). Thus, larger and more preserved samples were collected with the 19G needle, allowing a diagnosis of granulomas with a greater confidence than with the 22G needle.
Stoy et al. (33) investigated the success rate of PD-L1 testing from cytology cell block samples obtained by EBUS-TBNA and whether there were differences in specimen adequacy acquired via needles of different gauges. Out of 22 patients, PD-L1 was successfully tested in 20 cases, material was acquired with a 19G needle in 18.2% of samples, a 21G needle in 9.1% of samples, a 22G needle in 45.5% of samples, and a 25G needle in 27.2% of samples. There was no statistical difference in PD-L1 test success rates between needles with different sizes. Also, Herath (34) found the 19G needle useful to detect the PD-L1 expression, because the biopsies showed architectural details, underlining the importance of introducing new techniques to optimize the procedures.
Biswas et al. (35) described the yield of the 22G needle in providing adequate tissue for all genetic analysis and PD-L1 analysis in NSCLC: ancillary tests were successfully performed in the 82% of cases.
Gnass et al. (36) retrospectively evaluated 22 patients with hilar and/or mediastinal adenopathy sampled with a 19G needle diagnostic. The overall diagnostic yield was 100% for malignant and benign conditions, and in 12 of 14 cases of lung cancer the specimens were suitable for immunohistochemical and molecular staining (Table 2). Also, in this case the safety profile of the procedure was considered acceptable. This study underlined the operator satisfaction in the procedure, due to the better flexion of loaded scope and the good sample adequacy. The performers’ satisfaction was investigated also by Sczaniecka et al. (37) eight physicians were asked to rate the needles’ performance, as absolute performance and as in comparison with the currently available 21G and 22G needles, considering the penetration, the visibility, and the sample collection. For relative performance, physicians rated the 19G needle as comparable or better on 97.5% of the forms for sample collection, 92.5% for penetration, and 95% for visibility. For absolute performance, physicians rated the ability of the device to collect samples, its penetration, and its visibility as acceptable 98.75%, 97.5%, and 97.5% of the time respectively. However, it is obvious that the satisfaction of the operator is not a meaningful quality target for the needle.
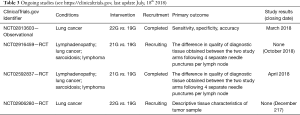
To sum up, with the available evidences it is difficult to make a sure conclusion in respect to when the 19G needle should be preferred; four randomized clinical trials are ongoing investigating the 19G flexible EBUS needle in comparison with the conventional 21G and 22G needles (Table 3) and we must wait for these studies before we decide when to use the 19G needle.
Miniforceps needles
The transbronchial miniforceps needle combines the sharpness of a needle, in order to go through the bronchial wall, and the presence of forceps to grab the target tissue for histological analysis. One of the first studies with miniforceps needle was performed by Herth et al. (38): 50 patients with enlarged or PET-positive mediastinal lymph nodes were included. In two cases penetration of the tracheobronchial wall was not possible. Of the remaining 48 cases, three samples were not adequate for the analysis and 2 were adequate but non-diagnostic. A diagnosis was made in 43 patients (31 cases of lung cancer, 6 cases of lymphoproliferative disorders, 2 tuberculosis, 4 sarcoidosis). No severe complications were observed. Franke et al. (39) compared 22G EBUS-needle with 21G EBUS miniforceps needle. Both needles were used in the same patient and the combination of the two techniques increased the diagnostic yield from 50% to 82%. Similar results were found by Darwiche et al. (40): the miniforceps increased the overall diagnostic yield for every condition and the diagnostic yield from 64% with EBUS-TBNA to 93% in benign conditions. All samples but one was adequate for molecular analysis. Chrissian et al. (41) suggested those results, reporting an overall diagnostic yield of 81% for EBUS-TBNA and 91% for miniforceps and when the techniques were combined the overall diagnostic yield was of 97%, significantly higher compared to EBUS-TBNA alone. It must be underlined that while in the study of Franke et al. (39) is described that the miniforceps needle has a 21G diameter, in the other studies the diameter of miniforceps needle is not described (19G in Darwiche et al. (40); 1 mm in Chrissian et al. (41).
On the other hand, Wang et al. (42) found, in a cohort of 227 patients, that the diagnostic yield of standard EBUS-TBNA cytology was comparable with miniforceps needle, indicating a non-superiority of the miniforceps biopsies. In conclusion, the mini-forceps needle seems promising in selected patients.
Future perspectives
Bramley et al. (43) investigated the role of a cautery-assisted transbronchial forceps biopsy (ca-TBFB) in 50 patients with mediastinal lymphadenopathies. Compared to the conventional EBUS-TBNA performed to 22G, the ca-TBFB performed better in granulomatous processes than in malignancy, with a sensitivity of 90% and 78% respectively (33% and 100% with the conventional EBUS-TBNA). Franke et al. (44) reported their experience with a cryo-needle, that has the advantage to provide material for histological evaluation. They obtained high-quality specimens, without artifacts, especially in those cases when a longer activation time was used.
In the future, in order of tapering the correct procedure on the correct patient, further studies are needed to elucidate the role of the needle size in the diagnostic process and in the subtyping of the various diseases and a more robust evidence for the use of larger bore needles from the ongoing trials are expected.
Conclusions
The current studies offer solid basis for further studies on the significance of the needle type for EBUS-TBNA and EUS-B-FNA in lung cancer and other diseases. Larger bore needles seem to be of value especially in histopathological assessment of benign diseases and lymphoproliferative disorders. Promising results are expected with 19G flexible needles, particularly in terms of diagnostic yield, cytological evaluation, histological evaluation, material for immunohistochemistry and material for molecular testing. Finally, also EBUS miniforceps needles were found useful in histological and molecular tests, notably when added to the EBUS fine needle biopsy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. Erratum in: Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). [Endoscopy 2015]. [Crossref] [PubMed]
- Colella S, Clementsen PF, Gurioli C, et al. Endobronchial-ultrasound needle aspiration and endoscopic ultrasound-fine-needle aspiration in thoracic diseases. Pathologica 2016;108:59-79. [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- van der Heijden EH, Casal RF, Trisolini R, et al. World Association for Bronchology and Interventional Pulmonology, Task Force on Specimen Guidelines. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [Crossref] [PubMed]
- Pöll JS. Historical note. The story of the gauge. Anaesthesia 1999;54:575-81. [Crossref] [PubMed]
- Duerig T, Pelton A, Stöckel D. An overview of nitinol medical applications. Materials Science and Engineering: A 1999;273-275:149-60. [Crossref]
- Keehan E, Cavanagh C, Gergely V. Novel alloy for speciality needle applications. Med Device Technol 2009;20:23-4, 26-7. [PubMed]
- Tyan C, Patel P, Czarnecka K, et al. Flexible 19-Gauge Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Needle: First Experience. Respiration 2017;94:52-7. [Crossref] [PubMed]
- 22-gauge ProCore Needle v. Standard 22-gauge (P00030500). ClinicalTrials.gov Identifier: NCT02154698
- Gounant V, Ninane V, Janson X, et al. Release of metal particles from needles used for transbronchial needle aspiration. Chest 2011;139:138-43. [Crossref] [PubMed]
- Dhillon SS, Yendamuri S. Needle assembly malfunction: an unusual complication related to endobronchial ultrasound-guided transbronchial needle aspiration. J Bronchology Interv Pulmonol 2013;20:252-5. [Crossref] [PubMed]
- Özgül MA, Çetinkaya E, Tutar N, et al. An unusual complication of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): the needle breakage. Ann Thorac Cardiovasc Surg 2014;20 Suppl:567-9. [Crossref] [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation (AQuIRE) Participants. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of theAmerican College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [Crossref] [PubMed]
- Jeyabalan A, Shelley-Fraser G, Medford AR. Impact of needle gauge on characterization of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014;19:735-9. [Crossref] [PubMed]
- Saji J, Kurimoto N, Morita K, et al. Comparison of 21-gauge and 22-gauge Needles for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration of Mediastinal and Hilar Lymph Nodes. J Bronchology Interv Pulmonol 2011;18:239-46. [Crossref] [PubMed]
- Rotolo N, Imperatori A, Nosotti M, et al. Multicentric study of endobronchial ultrasound-transbronchial needle aspiration for lung cancer staging in Italy. J Thorac Dis 2017;9:S370-5. [Crossref] [PubMed]
- Vaidya PJ, Saha A, Kate AH, et al. Diagnostic value of core biopsy histology and cytology sampling of mediastinal lymph nodes using 21-gauge EBUS-TBNA needle. J Cancer Res Ther 2016;12:1172-7. [PubMed]
- Izumo T, Sasada S, Watanabe J, et al. Comparison of two 22 G aspiration needles for histologic sampling during endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Jpn J Clin Oncol 2014;44:841-5. [Crossref] [PubMed]
- Dincer HE, Andrade R, Zamora F, et al. A new needle on the block: EchoTip ProCore endobronchial ultrasound needle. Med Devices (Auckl) 2016;9:467-73. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Randomized Study of 21-gauge Versus 22-gauge Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Needles for Sampling Histology Specimens. J Bronchology Interv Pulmonol 2011;18:306-10. [Crossref] [PubMed]
- Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol 2012;107:397-404. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Tremblay A, Yasufuku K, et al. A Preclinical Evaluation Comparing the Performance of a Novel 19-G Flexible Needle to a Commercially Available 22-G EBUS-TBNA SamplingNeedle. Respiration 2018;95:55-62. [Crossref] [PubMed]
- Garrison G, Leclair T, Balla A, et al. Use of an Additional 19-G EBUS-TBNA Needle Increases the Diagnostic Yield of EBUS-TBNA. J Bronchology Interv Pulmonol 2018;25:269-73. [Crossref] [PubMed]
- Chaddha U, Ronaghi R, Elatre W, et al. Comparison of Sample Adequacy and Diagnostic Yield of 19- and 22-G EBUS-TBNA Needles. J Bronchology Interv Pulmonol 2018;25:264-8. [Crossref] [PubMed]
- Tremblay A, McFadden S, Bonifazi M, et al. Endobronchial Ultrasound-guided Transbronchial Needle Aspiration With a 19-G Needle Device. J Bronchology Interv Pulmonol 2018;25:218-23. [PubMed]
- Ben S, Akulian J, Wang KP. Endobronchial ultrasound transbronchial needle aspiration: a hybrid method. J Thorac Dis 2015;7:S287-91. [PubMed]
- Trisolini R, Natali F, Ferrari M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration with the flexible 19-gauge needle. Clin Respir J 2018;12:1725-31. [Crossref] [PubMed]
- Balwan A. Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Using 19-G Needle for Sarcoidosis. J Bronchology Interv Pulmonol 2018;25:260-3. [Crossref] [PubMed]
- Biswas A, Wynne JP, Patel D, et al. Comparison of the yield of 19-G eXcelon core needle to a 21-G EBUS needle during endobronchial ultrasound guided transbronchial needle aspiration of mediastinal lymph nodes for the detection of granulomas in cases of suspected sarcoidosis. J Thorac Dis 2017;9:E864-6. [Crossref] [PubMed]
- Stoy SP, Rosen L, Mueller J, et al. Programmed death-ligand 1 testing of lung cancer cytology specimens obtained with bronchoscopy. Cancer Cytopathol 2018;126:122-8. [Crossref] [PubMed]
- Herath S, Cooper WA. The novel 19G endobronchial USS (EBUS) needle samples processed as tissue “core biopsies” facilitate PD-L1 and other biomarker testing in lung cancer specimens: case report and the view point from the Respiratory Physician and the Pathologist. Respirol Case Rep 2017;5:e00271. [Crossref] [PubMed]
- Biswas A, Leon ME, Drew P, et al. Clinical performance of endobronchial ultrasound-guided transbronchial needle aspiration for assessing programmed death ligand-1 expression in nonsmall cell lung cancer. Diagn Cytopathol 2018;46:378-83. [Crossref] [PubMed]
- Gnass M, Sola J, Filarecka A, et al. Initial Polish experience of Flexible 19-gauge Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Adv Respir Med 2017;85:64-8. [Crossref] [PubMed]
- Sczaniecka A, Gonzalez X, Tremblay A, et al. Performance of a Novel 19g EBUS-TBNA Needle in Patients. Chest 2016;150:1015A. [Crossref]
- Herth FJ, Schuler H, Gompelmann D, et al. Endobronchial ultrasound-guided lymph node biopsy with transbronchial needle forceps: a pilot study. Eur Respir J 2012;39:373-7. [Crossref] [PubMed]
- Franke KJ, Bruckner C, Szyrach M, et al. The contribution of endobronchial ultrasound-guided forceps biopsy in the diagnosticworkup of unexplained mediastinal and hilar lymphadenopathy. Lung 2012;190:227-32. [Crossref] [PubMed]
- Darwiche K, Freitag L, Nair A, et al. Evaluation of a novel endobronchial ultrasound-guided lymph node forceps in enlarged mediastinal lymph nodes. Respiration 2013;86:229-36. [Crossref] [PubMed]
- Chrissian A, Misselhorn D, Chen A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann Thorac Surg 2011;92:284-8. [Crossref] [PubMed]
- Wang JF, Baidoo C, Collins BT. Improved efficacy of endobronchial ultrasound-guided fine-needle aspiration biopsy in comparison to endobronchial ultrasound-guided miniforceps biopsy. Acta Cytol 2014;58:125-30. [Crossref] [PubMed]
- Bramley K, Pisani MA, Murphy TE, et al. Endobronchial Ultrasound-Guided Cautery-Assisted Transbronchial Forceps Biopsies: Safety and Sensitivity Relative to Transbronchial Needle Aspiration. Ann Thorac Surg 2016;101:1870-6. [Crossref] [PubMed]
- Franke KJ, Nilius G, Ruehle KH, et al. The cryo-needle: a new tool for histological biopsies. A feasibility study. Lung 2013;191:611-7. [Crossref] [PubMed]