
Effect of second primary cancer on the prognosis of patients with non-small cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer deaths around the world, and with the highest incidence are non-small cell lung cancers (NSCLCs) (1). With the improvement of diagnostic tools and the progress of treatment, more and more patients with NSCLC are surviving for a longer period of time ; thus, the chance of developing another cancer has become more and more prominent, which is distinguished from metastasis of the initial primary cancer (2,3).
Studies indicated that the incidence of multiple primary cancers (MPCs) ranges from 0.9% to 26.3% (2-7). Most of these studies focused on MPCs prior and after lung cancer and several researches had reported on the incidence of second primary cancers (SPCs) in NSCLC patients (8,9). However, up till present day, there are few studies that focused on SPC in NSCLC patients by using the Surveillance, Epidemiology, and End Results (SEER) database.
The aim of this study was to explore the incidence of SPCs in NSCLC patients by using the SEER database of the National Cancer Institute (NCI), and to investigate its effects on the survival of these patients.
Methods
We collected data in the SEER program of the NCI which covered approximately 28% of the US population (10). We used SEER*Stat version 8.3.4 software to extract data from the SEER database. This study was approved by the review board of our institute.
The selection criteria included patients who pathologically confirmed NSCLC between 2004 and 2014. For further refined screening, we selected patients with adenocarcinoma (SEER codes 8140, 8250, 8252–8255, 8260, 8310, 8323, 8480, 8481, 8490, 8570, 8574), squamous cell carcinoma (SEER codes 8052, 8070, 8074, 8083, 8084), large cell carcinoma (SEER codes 8012, 8013), and adenosquamous carcinoma (SEER code 8560). Patients with previous malignant diseases were excluded, and as well as patients without detailed information about SPC. Ultimately, 241,805 patients were included in this study.
The data that was extracted for this study included age, sex, race, marital status, if SPC occurred, histology, differentiation, surgical procedure, TNM stage [based on the criteria of the 6th edition of the American Joint Committee on Cancer (AJCC)], survival time, radiation and chemotherapy status. Age was grouped into four categories (≤60, 61–70, 71–80, and >80 years) in the following analyses. We classified surgical procedures into six groups: no surgery; sub-lobectomy (including local tumor destruction, excision or resection of less than one lobe such as wedge resection and segmentectomy); lobectomy (including lobe or bilobectomy); pneumonectomy; surgery (the type of operation is unclear), and unknown. Local tumor destruction included laser ablation or cryosurgery, electrocautery, fulguration (include the use of hot forceps for tumor destruction).
The criteria for multiple primary malignancies in the SEER registry were applied for those that were first proposed by Warren and Gates (11): each of the tumors must present a definite histologic picture of malignancy; each must be distinct; and if the probability that one was a metastatic lesion different from the other then must be excluded. Immunohistochemical staining was performed using cancer site specific antibodies if necessary (12).
The standards diagnosis of multiple primary lung cancer were as the following (13): tumors at different sites (for example, a tumor in a lung and another separate tumor in the trachea) or pathology; a single tumor in each lung; tumors diagnosed more than three years apart; an invasive tumor following an in situ tumor more than 60 days after the abstracted diagnosis as multiple primaries unless stated or proven to be metastatic; when it is not possible to determine if there is a single tumor or multiple tumors, abstract as a single primary; and tumors that do not meet any of the above criteria are a single primary. For cancers involving multiple organs, the diagnostic criteria for MPC are the same as indicated by Warren and Gates.
Statistics
The endpoint of this study was the overall survival (OS) and the lung cancer-specific survival (LCSS). The OS is the time interval from the initiation of the diagnosis of NSCLC to the death of patient from any causes. The LCSS is defined as the time interval from the diagnosis of NSCLC to the death from the primary NSCLC; and the death from any other causes is treated as censored. The association between variables was analyzed by either Chi-square test or Mann-Whitney and Wilcoxon test. Survival curve was generated using Kaplan-Meier method and the difference was examined using log-rank test. Cox regression analysis was performed to explore the significant predictors for OS and the hazard ratio (HR) of the prognostic factor was estimated. Because the time interval from the diagnosis of NSCLC to the occurrence of SPC varied among patients, so we examined the HR of SPC for patients who suffered from SPC within 6, 12, 36, 60 and 120 months, respectively. In the final Cox regression model, SPC was treated as a time-dependent covariate.
All statistical analyses were performed by SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA) and Stata/MP 14.0 for Windows (StataCorp LP, College Station, TX, USA). And a probability value less than 0.05 was considered to be significant.
Results
The patient cohort (n=241,805) in this study consisted of 130,166 males (53.8%) and 111,639 females (46.2%) with median age of 68.1 years (range, 5–105 years). The last follow-up time was December 2014, and mean follow-up time was 20 months (range, 0–131 months). During the follow up period, 179,304 (74.2%) patients died and median OS was 9 months. A total of 15,431 (6.4%) suffered from SPCs during the follow-up. The clinical characteristics of these patients are shown in Table 1.
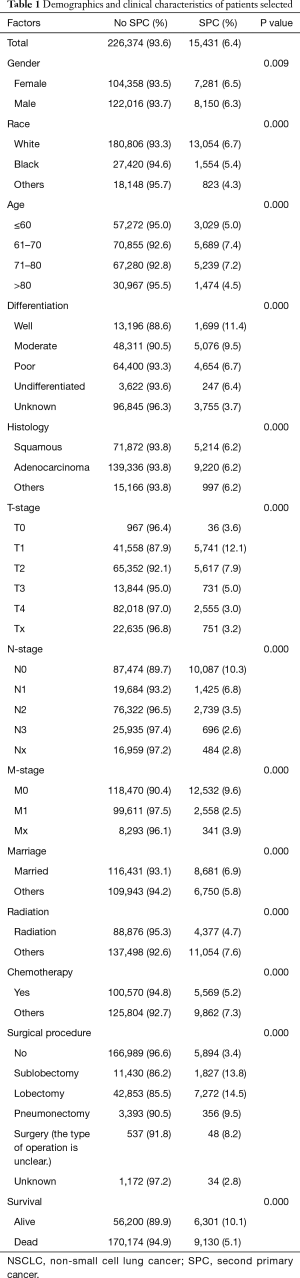
Full table
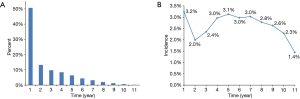
About half of the SPCs (50.7%) occurred during the first years after the diagnosis of NSCLC (Figure 1A). However, the incidences of SPC for the survived patients differed a little each year during the follow-up, fluctuating between 1.4% and 3.2% (Figure 1B). The median time interval from the diagnosis of NSCLC to the occurrence of SPC was 12 months (range, 0–128 months).
The most common SPC was lung cancer (45.1%), and the second one was colon and rectum cancer (6.9%), which was followed by prostate cancer (5.6%) and breast cancer (6.0%) (Figure 2A). The prognosis of patients suffered from prostate or breast cancer after diagnosis of NSCLC was better than those who suffered from the second primary lung cancer or colon and rectum cancer (median survival after diagnosis of SPC was 56, 48, 29 and 24 months for prostate cancer, breast cancer, lung cancer and colon and rectum cancer, respectively) (Figure 2B).

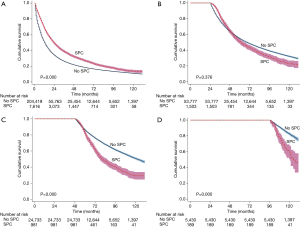
Patients with SPCs seemed to present a more favorable prognosis than those without SPCs (median survival 59 versus 13 months, P=0.000) (Figure 3). However, only those who survived for a period of time have the chance to suffer from SPC; thus, the interval from the time of diagnosis of NSCLC to the occurrence of SPC would be an immortal time bias. In order to reduce the immortal time bias, we compared patients’ survival who suffered from SPC during the first year, the third year, the fifth year and the ninth year after the diagnosis of NSCLC with those who survived to the same year without SPC. Results showed that patients who suffered from SPC were apt to have a poor prognosis as the time interval increases (Figure 4A,B,C,D).

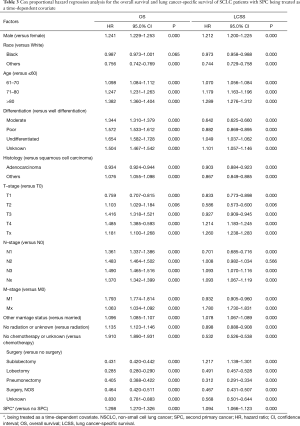
In the multivariable analysis with Cox regression model, we examined the HR for SPC after controlling confounders in patients who suffered from SPC within 6, 12, 36, 60 and 120 months, respectively. There was a persistent decline in HR with the increment of time span, from the time of diagnosis of NSCLC to the occurrence of SPC (HR diminished from 0.883 for those who suffered from SPC within 6 months, to 0.629 for those who suffered from SPC within 120 months) (Table 2). The proportional hazard hypothesis for SPC was false; thus, the final Cox regression model was fitted with SPC being treated as a time-dependent covariate. Our results showed that the occurrence of SPC was an adverse prognostic factor for patients with NSCLC [HR, 1.298; 95% confidence interval (CI), 1.270–1.326; P=0.000], and at the same time it increased the risk of LCSS (HR, 1.094; 95% CI, 1.066–1.123; P=0.000) (Table 3). Other significant prognostic factors for OS and LCSS included gender, age, differentiation, histology, TNM-stage, radiation, chemotherapy, and surgical procedure.

Full table
Full table
Discussion
With the advanced development in the diagnosis and treatment methods, many patients with NSCLC tend to have the chance to survive for a longer period of time. The occurrence of SPC in NSCLC patients, especially for long time survivors, is not a rare event. Several researches about SPC have been performed, focusing on the incidence, risk factors and survival and the incidence of SPC which ranged from 1.7% to 7.3% (3,4,6,8).
A study using data from SEER database between 1973 and 2003 by Hayat et al. had demonstrated the incidence of MPCs, which occurred before or after the primary lung cancer and it was 9% (14). In our current study, the incidence of SPC after NSCLC was lower than Hayat et al., and the probable reason might be because we excluded patients with history of malignant disease. The highest morbidity of SPCs after the diagnosis of NSCLC was lung cancer (45.1%), which might be due to the risk factors that cause lung cancer still exist (15). The second most common SPC was prostate cancer followed by colon and rectum cancer, which was similar to the incidence of these cancers in the general population (1). The survival time after the occurrence of prostate cancer was better than in breast cancer patients, followed by second primary lung, colon and rectum cancer. It was just similar to those who suffered from these cancers as their unique primary cancer (1). Similar to previous studies (4,6), about 50% of all SPCs occurred during the first year after the diagnosis of NSCLC. However, the incidence of SPC during each year after NSCLC being diagnosed had changed slightly, from 3.2% to1.4%. The findings indicate that attention should be paid more to the occurrence of SPC during all the follow-up period after the diagnosis of NSCLC.
The effect of SPC on the survival has been explored in several previous studies. In a retrospective study by Duchateau and Stokkel (3), multi-primary cancers either in their history or in the follow-up period were evaluated in 860 patients with NSCLC. The 5-year survival rate in patients with a second tumor in the follow-up period was better than in patients without any other second tumor (P=0.029). They speculated that patients with two or more cancers are predisposed to have rather slow progression tumors. However, we believe, only those who survived long enough had the opportunity to suffer from another primary cancer and this might inflicted the bias on the results favoring those with SPC.
In another retrospective study by Aguilo et al. (2), the OS of 1,686 patients with lung cancer as unique primary malignancy was compared with 228 patients who suffered from lung cancer and another independent primary cancer. Their results showed that patients with multiple cancers presented a slightly better survival rate than in patients with lung cancer as a unique primary cancer. They speculated that a second independent cancer should be considered as a separate entity in terms of prognosis and treatment. It seems that both cancers barely interact, and that it is the cancer with worst prognosis is the one which ultimately determines the outcome.
In this current study, with the exclusion of patients with previous malignant disease, the median survival time for patients with SPC was longer than those without SPC (59 months for SPC versus 13 months for no SPC, P=0.000) (Figure 3). Our results were similar to the previous studies (2,3,5). However, the time interval from the diagnosis of NSCLC to the occurrence of SPC was an immortal time during the survival analysis and it biased the results (16). In order to reduce the immortal time bias, we compared patients’ survival who suffered from SPC during the first year, the third year, the fifth year and in the ninth year after the diagnosis of NSCLC with those who survived to the same year without SPC (Figure 4A,B,C,D). For patients with synchronous SPC (occurrence at 0-6 months after diagnosis of NSCLC), a better prognosis was noted than those without SPC (Table 2), which is similar to the findings by Aguilo et al. (2). Patients with SPC were apt to have a poor prognosis with the increment of time interval between NSCLC and SPC. The reasons underlying the findings were unclear. However, with the increment of the time interval between NSCLC and SPC, patients with and without SPC inclined to be balanced with their baseline characters; however, more studies are needed regarding this issue.
In this study, Cox regression analysis was used to examine the HR of SPC, which occurred within 6, 12, 36, 60 and 120 months, respectively. Results showed that the HR decreased as the time span increased. The results indicated a false proportional hazard hypothesis; thus, a time-dependent Cox regression model was fitted with SPC being treated as a time-dependent covariate (17). Unlike the previous studies, in this current study SPC had demonstrated a significant prognostic factor detrimental to the survival in the Cox regression analysis (HR, 1.298; 95% CI, 1.270–1.326; P=0.000). To our knowledge, this is the first time to establish the adverse effects of SPC on the survival of NSCLC patients.
Different to our analysis, Donin et al. (18) analyzed cancer data of the SEER database from another aspect. They examined mortality that is attributable to first and second primary malignancies, and concluded that a significant proportion of cancer survivors will develop and die from lung cancer, which is consistent with the known epidemiology of lung cancer that it is the leading cause of cancer-related death in the world (1). Different from their research aspect, we studied the effect of SPC on the OS and LCSS of patients and concluded that the occurrence of SPC increased the risk of OS (29.8%) and LSCC (9.4%) separately for patients with NSCLC. Obviously, it has a much greater risk of non-LCSS death than the no SPC patients, but the reason is not entirely clear. However, NSCLC patients’ immunity might be influenced by SPC, which might result in more deaths.
Since the incidence of SPC is high and it affects patients’ survival with lung cancer, it is suggested that we should pay more attention to the screening of SPCs, especially second primary lung cancer. According to the NCCN guidelines for lung cancer screening (19), it is suggested that a history of prior lung cancer places an individual at higher risk for the development of a second lung cancer, irrespective of age and smoking history, and all lung cancer patients should be screened for second primary lung cancer. But, the information about the methods of screening of SPC is absent in the SEER database.
There are several potential limitations in our study that should be considered. Firstly, the presence of the heterogeneity of the population and the retrospective setting of this study is one of the limitations. Secondly, the SEER database itself is a limitation since it is an observational data that may engender inaccurate results (20). Thirdly, there was no information regarding comorbidities, targeted therapy, neoadjuvant therapy, and immune therapy in SEER database. This may have caused a certain bias in our results. Fourthly, the performance status and comorbidities of patients was unknown, which may have also affected the interpretation of our findings.
In conclusion, this study, despite the presence of several limitations, with the large number of patients had demonstrated the adverse effects of SPC on the survival rate using time-dependent Cox regression analysis. Attention should be paid to the screening of SPCs, especially the second primary lung cancer during the follow-up period. It is imperative that further investigations are needed to advance our understanding and to enable the development of effective measures against SPCs.
Acknowledgements
We would like to thank Dr. Hassan Dib for his contribution in proofreading of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethics Committee of Beijing Huaxin Hospital {[2018] ethical approval (37)}.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Aguilo R, Macia F, Porta M, et al. Multiple independent primary cancers do not adversely affect survival of the lung cancer patient. Eur J Cardiothorac Surg 2008;34:1075-80. [Crossref] [PubMed]
- Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest 2005;127:1152-8. [PubMed]
- Teppo L, Salminen E, Pukkala E. Risk of a new primary cancer among patients with lung cancer of different histological types. Eur J Cancer 2001;37:613-9. [Crossref] [PubMed]
- Brock MV, Alberg AJ, Hooker CM, et al. Risk of subsequent primary neoplasms developing in lung cancer patients with prior malignancies. J Thorac Cardiovasc Surg 2004;127:1119-25. [Crossref] [PubMed]
- Chuang SC, Scelo G, Lee YC, et al. Risks of second primary cancer among patients with major histological types of lung cancers in both men and women. Br J Cancer 2010;102:1190-5. [Crossref] [PubMed]
- Son C, Lee SK, Choi PJ, et al. Characteristics of additional primary malignancies in Korean patients with non-small cell lung cancer. J Thorac Dis 2013;5:737-44. [PubMed]
- Jegu J, Colonna M, Daubisse-Marliac L, et al. The effect of patient characteristics on second primary cancer risk in France. BMC Cancer 2014;14:94. [Crossref] [PubMed]
- Tabuchi T, Ito Y, Ioka A, et al. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci 2012;103:1111-20. [Crossref] [PubMed]
- Howlader N, Krapcho M, Miller D, et al. SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission. Posted to the SEER web site April 2017.
- Warren S, Gates O. Multiple primary malignant tumors. A survey of the literature and statistical study. Am J Cancer 1932;16:1358-64.
- Hashimoto K, Sasajima Y, Ando M, et al. Immunohistochemical profile for unknown primary adenocarcinoma. PLoS One 2012;7:e31181. [Crossref] [PubMed]
- Johnson CH PS, Adamo P, Fritz A, et al. The 2007 Multiple Primary and Histology Coding Rules. National Cancer Institute, Surveillance, Epidemiology and End Results Program. Bethesda, MD, 2007.
- Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007;12:20-37. [Crossref] [PubMed]
- Abdel-Rahman O, Cheung WY. Subsequent thoracic cancers among patients diagnosed with lung cancer: a SEER database analysis. Curr Med Res Opin 2017;33:2009-17. [Crossref] [PubMed]
- Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int 2018;31:125-30. [Crossref] [PubMed]
- Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013;31:2963-9. [Crossref] [PubMed]
- Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016;122:3075-86. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology-Lung canser screening. V.3.2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf
- Giordano SH, Kuo YF, Duan Z, et al. Limits of observational data in determining outcomes from cancer therapy. Cancer 2008;112:2456-66. [Crossref] [PubMed]


