
Mesenchymal tumors of the lung: diagnostic pathology, molecular pathogenesis, and identified biomarkers
Introduction
Lung cancers are primarily composed of epithelial tumors such as carcinomas. As mesenchymal tumors in the lung are very rare, they have garnered little attention. The 2015 World Health Organization (WHO) classification of lung tumors has undergone revision, not only for carcinomas but also for mesenchymal tumors (1,2); for example, PEComatous tumors, myoepithelial tumors, and pulmonary myxoid sarcomas with EWSR1-CREB1 translocation have been adopted as new disease entities (1,2). To date, no review articles have comprehensively summarized what is known about pulmonary mesenchymal tumors in accordance with the latest WHO classification. We present current data about these tumors (except for pediatric tumors), with a special emphasis on their diagnostic pathology, molecular pathogenesis, and identified biomarkers, in line with the 2015 WHO classification. We also describe recently recognized pulmonary mesenchymal tumors that have not yet been included in the WHO classification (Table 1).
Full table
Vascular tumors and vessel-associated tumors
Blood vessels, including alveolar blood capillaries, are a prevalent source of mesenchymal elements in the lung. The vasculature is composed not only of endothelial cells but also of other mesenchymal cells such as intimal mesenchymal cells and perivascular cells. For the diagnoses of vascular and vessel-associated tumors, in addition to hematoxylin and eosin (HE) staining, immunohistochemical and molecular techniques are indispensable. We describe below the diagnostic pathology and molecular biomarkers of these tumors.
Epithelioid hemangioendothelioma
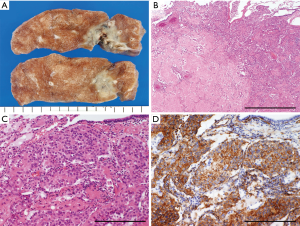
Epithelioid hemangioendotheliomas are low- to intermediate-grade malignant vascular tumors (Figure 1) (1,3,4). Pulmonary epithelioid hemangioendotheliomas typically appear as multiple nodules with an intra-alveolar architecture and central region of hyalinization (Figure 1B) (3,5). Microscopically, tumor cells are characterized by centrally located, round-to-ovoid nuclei and intracytoplasmic lumina that may contain erythrocytes. These tumors form nodules with short strands or solid nests (2,3). Immunohistochemically, these tumors stain positive for endothelial markers (e.g., CD31, CD34, and factor VIII; Figure 1D) (3,4). Because of their occasional expression of cytokeratin, epithelioid hemangioendotheliomas may be misdiagnosed as carcinomas or mesotheliomas (3,4). Genetically, epithelioid hemangioendotheliomas usually harbor a WWTR1-CAMTA1 fusion gene, which is not observed in angiosarcomas (3,4). A recent report demonstrated the presence of a YAP1-TFE3 fusion gene in a subset of young adults with these tumors (6).
Pulmonary artery intimal sarcoma
Pulmonary artery intimal sarcomas originate from the arterial intima of elastic-type arteries in the lung (2). They grow in intraluminal areas and spread along the intima, sparing the media of the vessel (7). In patients in which these tumors protrude into arterial lumens, pulmonary embolisms or pulmonary hypertension may develop (7,8). Microscopically, pulmonary artery intimal sarcomas typically exhibit a heterogeneous morphology but may be completely undifferentiated or heterologous (7,9).
Pulmonary artery intimal sarcomas do not have a specific immunophenotype (7,9,10). Genetically, most harbor an MDM2 amplification, which often coexists with a PDGFRA amplification that can often be used as a druggable target (7,10). However, these genetic alterations are not specific to these tumors. Taken together, no specific biomarker is available for their diagnosis.
Glomus tumor
Glomus tumors rarely arise in the lung (Figure 2); most develop in distal extremities, especially in subungual regions (11). Glomus cells are primarily located in the reticular dermis of the acral skin and function as a thermal regulator (12). Glomus tumor cells resemble the smooth muscle cells of the glomus body (11). Pulmonary glomus tumors are usually indolent but frequently involve the bronchus and trachea (13,14).
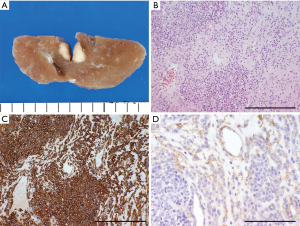
Microscopically, glomus tumors, which are composed of round, epithelioid cells with centrally located nuclei and light eosinophilic cytoplasm, form clusters that surround small capillary-size vessels (Figure 2B) (11).
Immunohistochemically, these tumors usually stain positive for α-smooth muscle actin (α-SMA) and often for other smooth muscle cell markers (e.g., h-caldesmon, calponin, and desmin; Figure 2C) (13,15,16). However, these markers are not specific to glomus tumors. Mesh-like staining of the intercellular material for type IV collagen is a common characteristic (Figure 2D).
According to their malignant potential, glomus tumors are classified into three subgroups: benign, uncertain malignant potential, and malignant (11). Most belong to the benign subgroup (11). Malignant glomus tumors are defined as tumors harboring all of the following malignant features: (I) marked nuclear atypia; (II) high mitotic activity (≥5 mitoses/50 HPF); and (III) atypical mitotic figures (11,15)). Glomus tumors with uncertain malignant potential are defined as tumors harboring one or two of the above-mentioned malignant features. These pathological findings are used to predict the malignant potential of glomus tumors. A recent report found that 16% of malignant glomus tumors or glomus tumors with uncertain malignant potential harbor a BRAFV600E mutation (17). The BRAFV600E mutation may thus serve not only as a prognostic biomarker but also as a molecular target, given the efficacy of BRAF-targeted therapies against neoplasms with BRAF mutations (18).
PEComatous tumor
Perivascular epithelioid cell tumors (PEComas) appear as a localized mass composed of perivascular epithelioid cells with clear-to-pale eosinophilic cytoplasm. Pulmonary PEComatous tumors are classified into three subgroups: PEComas, lymphangioleiomyomatosis, and tumors with overlapping features of these two subgroups (Figure 3) (2). Renal or hepatic angiomyolipomas are also categorized as PEComatous tumors.
Immunohistochemically, PEComatous tumors stain positive for estrogen receptor (ER; Figure 3B), melanocyte markers (e.g., HMB45, Melan A, and S100; Figure 3C), and smooth muscle cell markers. The positive expression of melanocyte and ER markers is a diagnostic clue for the differentiation of PEComatous tumors, especially lymphangioleiomyomatosis, from diffuse pulmonary lymphangiomatosis and metastatic sarcomas (see below). Pulmonary PEComas frequently harbor a TSC mutation (2). A TFE3 rearrangement has been identified in a subset of PEComas located in the gynecologic tract, which are responsive to mTORC1 inhibitors (2,19,20).
Diffuse pulmonary lymphangiomatosis
Diffuse pulmonary lymphangiomatosis usually develops in children and, less frequently, adults (2,21,22). This disease exhibits diffuse proliferation of lymphatic vessels and smooth muscle cells (2,21,22). Immunohistochemically, the proliferating lymphatic vessels stain positive for lymphatic endothelial markers (e.g., D2-40, CD31, and factor VIII) (21,22). Diffuse pulmonary lymphangiomatosis stains negative for ER and HMB45, which are both expressed in PEComas. The combination of lymphatic endothelial markers and ER and HMB45 markers is useful to differentiate diffuse pulmonary lymphangiomatosis from PEComas.
Solitary pulmonary capillary hemangioma (SPCH)
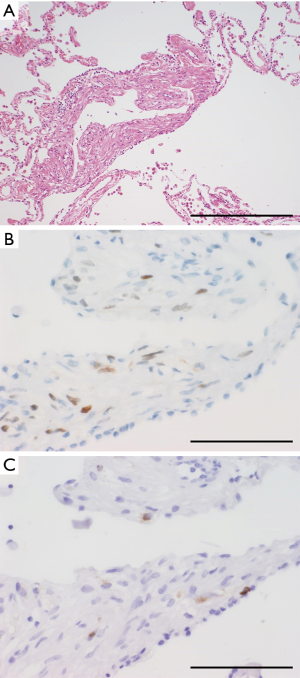
SPCH is a rare, benign, mesenchymal tumor that is characterized by the proliferation of capillaries (Figure 4) (23-25). SPCH is not included in the current WHO classification (2). “Sclerosing hemangioma,” which is not a hemangioma and has been replaced by “sclerosing pneumocytoma” in the current WHO classification, requires distinction from SPCH. In older reports, sclerosing pneumocytoma was mistakenly described as hemangioma because of the misuse of these terms (25). As with the case of sclerosing pneumocytomas, SPCHs do not show any symptoms and are usually identified coincidentally on computed tomography (CT) for other medical reasons (25). SPCH appears as a ground-glass nodule (GGN) or part-solid GGN on chest CT; therefore, the differential diagnosis between SPCH and small-size peripheral adenocarcinoma is a common challenge in radiology (25). Thus, SPCH should be included in the differential diagnosis of GGN lesions (25). The malignant transformation of SPCH has never been reported; accordingly, this tumor is not considered a precursor of malignant vascular tumors such as angiosarcoma or pulmonary artery intimal sarcoma. SPCHs do not require surgical removal, although a preoperative diagnosis is challenging.
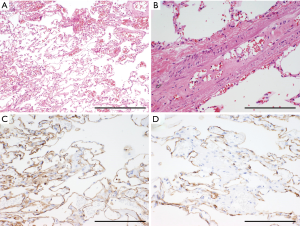
Macroscopically, SPCH usually presents as a brownish lesion that resembles focal congestion or a hematoma. Its macroscopic appearance makes the lesion difficult to identify in both raw and formalin-fixed specimens (24,25). Microscopically, SPCH sections reveal the proliferation of dilated capillaries within alveolar septa (Figure 4A) (25). Protrusion of the proliferating capillaries into small vessels is one of their characteristic features (Figure 4B) (25,26). Markedly dilated capillaries often mimic alveolar lumens; in such cases, positive immunostaining for cytokeratin in alveolar septa serves as a diagnostic clue (Figures 4C,D) (25). Immunohistochemically, proliferating capillaries stain positive for both endothelial markers (e.g., CD31, CD34, and factor VIII; Figure 4C) and α-SMA, whereas alveolar capillaries usually do not express α-SMA (25). The expression of α-SMA may reflect the existence of pericytes (25), which is a characteristic of, but not specific to, SPCH. The molecular pathogenesis of these tumors is unclear, and currently, they are diagnosed only by morphological and immunohistochemical findings. As reactive angiogenesis was mistakenly diagnosed as SPCH in several reports because of histological similarities, the differentiation of reactive angiogenesis from SPCH should be confirmed.
Nonvascular spindle cell tumors
The most prevalent subset of mesenchymal tumors is composed of spindle cells. Because of the shared morphological features of many tumor types, including vascular spindle cell tumors, the differential diagnosis of spindle cell tumors is difficult if only based on morphological findings. For a correct differential diagnosis, immunohistochemical and molecular techniques are indispensable. We describe below the nonvascular spindle cell tumors, focusing on their immunohistochemical and molecular markers.
Intrapulmonary solitary fibrous tumor (SFTs)
SFTs (also known as hemangiopericytomas) belong to the class of fibroblastic tumors that rarely arises in the lung. SFTs typically develop in the pleura as well as in extra-pleuropulmonary organs, especially the meninges (11,27). Intrapulmonary SFTs share similar characteristics with extrapulmonary SFTs (Figure 5) (28).
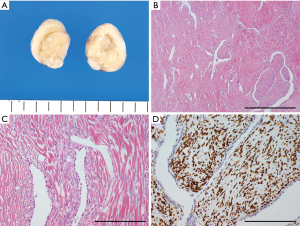
Histologically, SFTs are composed of fibroblastic spindle cells and show characteristic patternless architectures of both hypo- and hypercellular areas, along with collagenous fibers. SFTs contain branching, thin-walled, hemangiopericytomatous (staghorn) vessels (Figure 5C) (11,27). Intrapulmonary SFTs occasionally appear adenofibromatous because of the entrapment of pulmonary epithelium (Figure 5B) (28). Immunohistochemically, SFTs stain positive for CD34, BCL2, and CD99; however, these markers are not specific to this tumor type. Recently, STAT6 emerged as a highly specific immunohistochemical marker of SFTs (Figure 5D) (29,30). The expression of STAT6 results from an NAB2-STAT6 fusion gene, which is specifically observed in SFTs (29-32). The fusion gene involving STAT6 and the subsequent expression of STAT6 protein are characteristically observed in SFTs and serve as reliable diagnostic markers.
SFTs usually behave as benign tumors; however, local recurrence or metastasis is observed in approximately 5–20% of all SFTs (33). Recent studies reported that a size greater than or equal to 10 cm, severe cellular atypia, tumor necrosis, greater than or equal to 4 mitoses/10 HPF, and a Ki-67 labeling index greater than or equal to 2% are all associated with a high risk of recurrence of pleuropulmonary SFTs (33-35). However, a subset of intrapulmonary SFTs without these features was shown to undergo recurrence or metastasis (28). It remains challenging to identify the malignant potential of these tumors.
Inflammatory myofibroblastic tumor (IMT)
IMTs usually harbor a low malignant potential and typically develop in children or young adults (2,36). IMTs usually arise in soft tissues, such as the abdomen and pelvis, but occasionally develop in the lung (36).
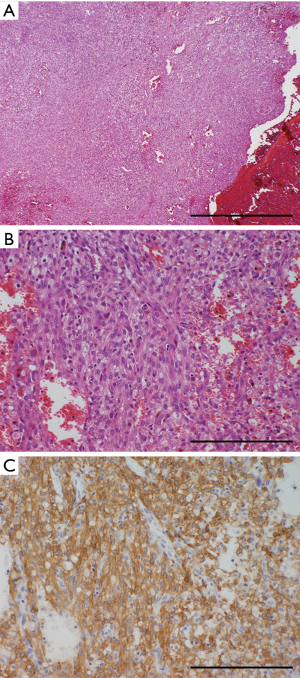
Microscopically, IMTs are composed of abundant inflammatory lymphoplasmacytes and spindle cells that proliferate in a fascicular pattern (Figure 6) (2,36). Immunohistochemically, these tumors stain positive for α-SMA, which is a characteristic of, but not specific to, IMTs and reflects their differentiation from myofibroblasts. Immunostaining for ALK is relatively specific; however, about 50% of cases stain negative for ALK, making this marker less sensitive as a diagnostic biomarker (36). The recently developed immunohistochemical intercalated antibody enhanced polymer (iAEP) method can increase the sensitivity of ALK staining, in which previously ALK-negative IMTs are reclassified as positive (Figure 6C) (37). Genetically, IMTs frequently harbor an ALK gene rearrangement, which can be confirmed by fluorescence in situ hybridization (FISH) or reverse transcription-polymerase chain reaction (RT-PCR) (37,38). Because ALK inhibitors are promising drugs to target ALK-rearranged neoplasms (37,38), the ALK fusion gene may serve as a companion diagnostic marker for malignant IMTs.
Synovial sarcoma
Despite its name, a synovial sarcoma does not undergo differentiation into synovial tissues (2). These tumors usually arise in soft tissues, such as those of the extremities. Except for these soft tissue regions, the lung is one of the most common organs in which synovial sarcomas arise and to which they metastasize (2,11,39). According to the degree of epithelial differentiation, these tumors are classified into three subtypes: monophasic, biphasic, and poorly differentiated (2,11). Most pulmonary synovial sarcomas belong to the monophasic and poorly differentiated subtypes (39-42).
Pulmonary synovial sarcomas frequently exhibit a poorly differentiated morphology and undergo a highly aggressive clinical course compared to synovial sarcomas in soft tissues (41,42). Immunohistochemically, these tumors stain positive for CD99 and BCL2, but these markers are not specific to this tumor (41,42). Synovial sarcomas can stain positive for epithelial markers, albeit to various extents (e.g., cytokeratin and epithelial membrane antigen), especially the biphasic subtype (41,42). A recent study found that TLE1 is a highly specific immunohistochemical marker for these tumors (41,43). Genetically, t(X;18)(p11;q11), which leads to an SYT-SSX fusion, is considered a specific translocation that is diagnostic of synovial sarcomas by FISH or RT-PCR (44). Recently developed vaccine therapies against the SYT-SSX fusion-derived peptide have shown efficacy for the treatment of synovial sarcomas (45). The SYT-SSX fusion gene may serve as a companion diagnostic marker for these tumors.
Pulmonary myxoid sarcoma with EWSR1-CREB1 translocation
Pulmonary myxoid sarcoma with EWSR1-CREB1 translocation is a recently recognized malignancy (1,2). Microscopically, this tumor shows evidence of spindle or satellite cell proliferation accompanied by myxoid stroma. Currently, there are no immunohistochemical markers that support the diagnosis of this tumor.
Genetically, this sarcoma harbors an EWSR1-CREB1 fusion gene (46); however, this fusion is not specific to this tumor as it is also observed in other neoplasms (e.g., angiomatoid fibrous histiocytoma and clear cell carcinoma of the soft tissue or gastrointestinal tract) (47). Detection of this fusion by FISH or RT-PCR serves as a diagnostic clue; however, histological findings are also indispensable for the diagnosis of these tumors. A recent study advocated the inclusion of intracranial myxoid mesenchymal tumor as a new disease entity (48). In these intracranial tumors, ATF1 and CREB1, which both belong to the CREB family, form a fusion gene with EWSR1 (48). If an EWSR1-ATF1 fusion is identified in a “pulmonary myxoid sarcoma with EWSR1-CREB1 translocation,” the tumor may be termed a “pulmonary myxoid sarcoma with EWSR1-CREB translocation”.
Other mesenchymal tumors and tumor-like lesions
In this section, we describe other mesenchymal tumors and tumor-like lesions. Recent studies showed that myoepithelial carcinomas share similar genomic features with sarcomas, although myoepithelial carcinoma had been considered an epithelial malignancy (49,50). Another study discussed the clonality of pulmonary hamartoma. Moreover, several tumor-like lesions in the lung must be distinguished from lung neoplasms.
Myoepithelial tumor/myoepithelial carcinoma
Pulmonary myoepithelial tumors are composed of proliferating myoepithelial cells (1,2). Immunohistochemically, these tumors express cytokeratin, α-SMA, calponin, p63, and S100 to various degrees. The immunoreactivity of these myoepithelial markers serves as a useful diagnostic clue (49). Genetically, pulmonary myoepithelial carcinomas harbor an EWSR1 gene rearrangement, as is the case for their salivary duct counterpart (49,50). Although myoepithelial carcinomas differ from epithelial tumors despite their name, these tumors genetically resemble sarcomas (49).
Pulmonary hamartoma
Hamartomas are composed of a disorganized arrangement of normal tissue elements. “Pulmonary hamartoma”, rather than just “hamartoma”, is the recommended disease term (1). Pulmonary hamartoma is composed of the abnormal proliferation of at least two mesenchymal elements. Most pulmonary hamartomas are composed of cartilaginous and connective tissues along with entrapped respiratory epithelium. The morphologic features of pulmonary hamartoma are sufficiently characteristic that immunohistochemical staining is unnecessary for its diagnosis (1).
Pulmonary hamartomas are true neoplasms, whereas most hamartomas arising in other sites are not considered neoplastic (1,2). The neoplastic nature of pulmonary hamartoma is demonstrated by the HMGA2-LPP fusion gene (51,52). The HMGA2 fusion in pulmonary hamartomas suggests that these tumors share a similar pathogenesis with other mesenchymal tumors that harbor an HMGA2-involved fusion gene (e.g., chondromas, extraskeletal osteochondromas, lipomas, and leiomyomas) (53-55). Chondromas histologically resemble pulmonary hamartomas and harbor a similar fusion gene in extrapulmonary regions. The entrapped bronchial epithelium in pulmonary hamartomas serves as a clue for the differentiation of chondromas from pulmonary hamartomas (54).
Chondroma
Chondromas are benign neoplasms that are solely composed of cartilaginous tissues without entrapment of the respiratory epithelium (2,56). Pulmonary chondromas are rare but comprise one of the features of the Carney triad (pulmonary chondroma, paraganglioma, and gastric stromal tumor) (56,57). In patients with the Carney triad, multiple nodules of the chondroma frequently develop in the periphery of the lung.
Genetically, although extrapulmonary chondromas often harbor an HMGA2-involved fusion gene, similar to that found in pulmonary hamartomas (51-55), pulmonary chondromas with such a fusion gene have not been documented. One report presented a gain of chromosome 6 and a loss of 1q in a pulmonary chondroma (57).
Granular cell tumor
Granular cell tumors, which show evidence of neural sheath differentiation, rarely develop in the lung (Figure 7) (58); the majority arise in the head and neck (11).
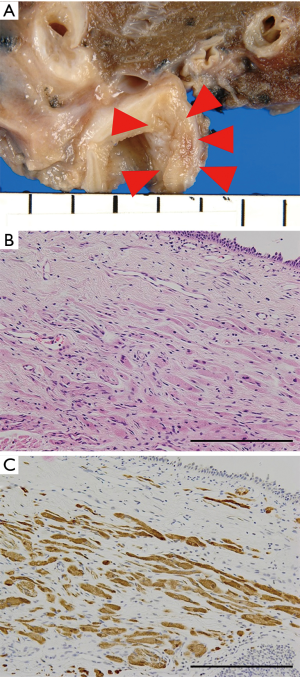
Pulmonary granular cell tumors frequently involve the bronchi and occasionally protrude into the bronchial lumen (Figure 7A); however, they typically undergo an indolent clinical course (58). Granular cell tumors consist of subepithelial oval cells with abundant eosinophilic granular cytoplasm (Figure 7B). The overlying epithelium may show evidence of pseudoepitheliomatous hyperplasia (59), mimicking squamous cell carcinoma. Intracytoplasmic granules are evident by periodic acid-Schiff staining with diastase.
Immunohistochemically, these tumors stain positive for both S100 (suggestive of neural cell differentiation) and CD68 (from intracytoplasmic phagolysosomes), which serve as diagnostic clues (Figure 7C) (59,60).
The majority of granular cell tumors are benign. Malignant granular cell tumors are characterized by increased mitotic activity (>2 mitoses/10 HPF), necrosis, marked cellular pleomorphism, and a high nuclear-to-cytoplasmic ratio (11,61).
Genetically, monosomy 22, trisomy 10, and loss of CDKN2A are frequently observed in malignant granular cell tumors, similar to malignant peripheral neural sheath tumors (MPNSTs) (11,62,63). Both malignant granular cell tumors and MPNSTs show evidence of the neural sheath differentiation and share similarities in molecular alterations. Malignant granular cell tumors and MPNSTs with granular cell changes share similar genetics and morphologies; therefore, their classification into independent disease entities remains controversial (61).
Nodular pulmonary amyloidosis
Nodular pulmonary amyloidosis presents as a tumor-like nodule, which must be distinguished from lung neoplasms. Pulmonary amyloidosis develops as a result of amyloid deposition and is classified into three subtypes according to the deposition pattern: diffuse alveolar septal, tracheobronchial, and nodular (64,65). A few reports have described multiple myeloma patients with pulmonary hypertension due to the deposition of AL-amyloid in large pulmonary vessels (66,67). Nodular pulmonary amyloidosis must be carefully distinguished from a true neoplasm.
In nodular pulmonary amyloidosis, AL-type amyloid is usually deposited in the lung in association with lymphoproliferative diseases (68). Morphologically, nodular amyloidosis can appear as a solitary nodule or as multiple nodules (68). Nodular amyloidosis appears amorphous and eosinophilic by HE staining and reddish-orange by direct fast scarlet or Congo red staining. Because light chain deposition disease also appears as a nodule and shares similar histological and staining patterns with nodular amyloidosis, their differentiation from one another is often difficult (69). Apple-green birefringence by polarization is specifically observed in amyloidosis, serving as a diagnostic clue. It should be noted that lymphoproliferative diseases (usually lymphoma), which frequently coexist with amyloidosis, must be appropriately diagnosed for determination of an optimal therapeutic strategy and clinical course.
IgG4-related disease (IgG4-RD)
IgG4-RD is a newly recognized inflammatory disease and is characterized by elevated levels of serum IgG4, IgG4-positive lymphoplasmacytic infiltration, and fibrosis (70-72). The diagnostic criteria for IgG4-RD are confusing due to the existence of two different criteria (Japan criteria and Boston criteria) (71,72). An increased number of IgG4-positive plasma cell infiltrations and high-IgG4/IgG ratios are both included in the two criteria (71,72); however, high serum IgG4 level is included in the Japan criteria, but not in the Boston criteria (71,72).
IgG4-RD is a systemic disease that occasionally involves the lung (70). Radiologically, this disease can appear as a solitary nodule or as an interstitial pneumonia-like shadow (73). To be exact, IgG4-RD falls outside of the category of mesenchymal tumors. However, IgG4-RD occasionally forms a tumor-like lesion with fibrous proliferation and IgG4-positive lymphoplasmacytic infiltrates. Radiologically, the differentiation of IgG4-RD from lung tumors is often challenging, even with positron emission tomography-CT (74).
Fibrotic proliferation is one of the morphological characteristics of IgG4-RDs and is included in the Boston criteria (70-72). IgG4-RD is highly responsive to glucocorticosteroids (70,75); therefore, this disease requires a correct diagnosis for determination of an optimal therapeutic treatment. When this disease appears with pulmonary fibrosis, lymphoplasmacytic infiltrates serve as a diagnostic clue. The presence of eosinophilic infiltrates is also a supportive finding in its diagnosis, although this factor is not included in the diagnostic criteria (70-72). Obviously, positive IgG4 immunostaining must be confirmed for the diagnosis of IgG4-RD.
Metastatic mesenchymal tumors
Blood flows through the venae cavae and into the right ventricle before circulating into the lung; various neoplasms, including mesenchymal tumors, metastasize to the lung. Because a variety of mesenchymal tumors are detected as pulmonary metastatic foci prior to the detection of primary lesions (76), metastatic tumors must be correctly diagnosed as early as possible for successful treatment.
Metastatic mesenchymal tumors usually share histological and immunohistochemical features with their primary tumors (Figure 8). These shared characteristics serve as diagnostic clues. Although metastatic mesenchymal tumors usually appear as solid masses, several types of metastatic tumors (e.g., leiomyosarcoma, synovial sarcoma, low-grade endometrial stromal sarcoma, and undifferentiated uterine sarcoma) occasionally appear as cystic lesions (Figure 8C) (76-80). This unusual appearance makes it difficult to diagnose them radiologically or pathologically.
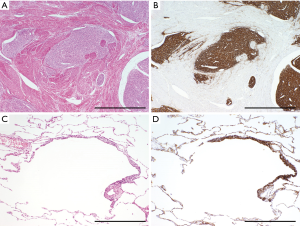
The term “cyst” is used in various clinical disciplines (77). In respirology, a cyst usually indicates a dilated airway, such as a bulla or lymphangioleiomyoma. Metastatic mesenchymal tumors of the bronchiole often serve as a check valve that subsequently causes cystic dilatation of the airway (76). Such tumors can histologically mimic lymphangioleiomyomas, which stain positive for HMB45 (Figure 3); however, these metastatic mesenchymal tumors usually stain negative for HMB45 and positive for immunohistochemical markers shared with their primary tumors (Figure 8). Therefore, these markers are useful for a differential diagnosis (80). On the other hand, in gynecology and gastroenterology, a cyst denotes a dilated structure that contains fluid in its lumen. Metastatic mesenchymal tumors may show such fluid-containing cysts with various accompanying changes (76). In such cases, congenital cysts, such as a bronchogenic cyst, must be included in the differential diagnosis. Radiologically, metastatic tumors with cystic structures may show water-density shadows on plain CT (77), which is not typical of metastatic tumors. In such cases, an enhanced cystic wall on contrast-enhanced CT supports a diagnosis of a metastatic tumor with a cystic structure. Microscopically, tumor cells are usually located on the cystic wall along with secondary changes, such as the accumulation of hemosiderin-laden macrophages, foamy cells, or osteoclast-like giant cells (76,77). To correctly interpret such immunohistochemical results, the differentiation of tumor cells from accumulated histiocytes is required.
Conclusions and future directions
Mesenchymal tumors rarely develop in the lung. Because of the scarcity of pulmonary mesenchymal tumors, to the best of our knowledge, no recent review article has comprehensively summarized them in contrast to epithelial tumors (81-83). The WHO classification of soft tissue and bone tumors categorizes mesenchymal tumors according to their mechanism of molecular pathogenesis (11). Similarly, pulmonary mesenchymal tumors will be reclassified according to their genomic features. An increased understanding of the molecular characteristics of pulmonary mesenchymal tumors has the potential to provide clinicians with the best therapeutic options for affected patients with the aid of newly identified biomarkers.
Acknowledgements
The authors thank Teppei Morikawa and Hajime Horiuchi for their academic assistance, Misato Nakajima, Goichiro Yanagi and Tomoyo Kakita for their excellent technical assistance, and Yuki Takano and Chikako Yoshida for their secretarial expertise.
Funding: This study was supported financially by JSPS KAKENHI Grant Number JP18K15103 (H Hashimoto) and JP16K08679 (K Inamura).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al, editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer, 2015.
- Weissferdt A, Moran CA. Primary vascular tumors of the lungs: a review. Ann Diagn Pathol 2010;14:296-308. [Crossref] [PubMed]
- Anderson T, Zhang L, Hameed M, et al. Thoracic epithelioid malignant vascular tumors: a clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol 2015;39:132-9. [Crossref] [PubMed]
- Woo JH, Kim TJ, Lee KS, et al. Epithelioid hemangioendothelioma in the thorax: Clinicopathologic, CT, PET, and prognostic features. Medicine (Baltimore) 2016;95:e4348. [Crossref] [PubMed]
- Antonescu CR, Le Loarer F, Mosquera JM, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 2013;52:775-84. [Crossref] [PubMed]
- Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch 2001;438:57-65. [Crossref] [PubMed]
- Mussot S, Ghigna MR, Mercier O, et al. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg 2013;43:787-93. [Crossref] [PubMed]
- Huo L, Moran CA, Fuller GN, et al. Pulmonary artery sarcoma: a clinicopathologic and immunohistochemical study of 12 cases. Am J Clin Pathol 2006;125:419-24. [Crossref] [PubMed]
- Dewaele B, Floris G, Finalet-Ferreiro J, et al. Coactivated platelet-derived growth factor receptor {alpha} and epidermal growth factor receptor are potential therapeutic targets in intimal sarcoma. Cancer Res 2010;70:7304-14. [Crossref] [PubMed]
- Fletcher CDM, Bridge JA, Hogendoorn P, et al, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon: International Agency for Research on Cancer, 2013.
- Kanik A, Li M, Urmacher CD. Normal skin. In: Mills SE. editor. Histology for Pathologists. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007:3-30.
- Gaertner EM, Steinberg DM, Huber M, et al. Pulmonary and mediastinal glomus tumors--report of five cases including a pulmonary glomangiosarcoma: a clinicopathologic study with literature review. Am J Surg Pathol 2000;24:1105-14. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Moran CA. Intrathoracic glomus tumors and glomangiosarcomas: a clinicopathological and immunohistochemical study of 14 cases with emphasis on anatomic distribution. Virchows Arch 2016;469:541-6. [Crossref] [PubMed]
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol 2001;25:1-12. [Crossref] [PubMed]
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol 2015;23:181-8. [Crossref] [PubMed]
- Karamzadeh Dashti N, Bahrami A, Lee SJ, et al. BRAF V600E Mutations Occur in a Subset of Glomus Tumors, and Are Associated With Malignant Histologic Characteristics. Am J Surg Pathol 2017;41:1532-41. [Crossref] [PubMed]
- Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642-50. [Crossref] [PubMed]
- Schoolmeester JK, Dao LN, Sukov WR, et al. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol 2015;39:394-404. [Crossref] [PubMed]
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 2010;28:835-40. [Crossref] [PubMed]
- Tazelaar HD, Kerr D, Yousem SA, et al. Diffuse pulmonary lymphangiomatosis. Hum Pathol 1993;24:1313-22. [Crossref] [PubMed]
- Boland JM, Tazelaar HD, Colby TV, et al. Diffuse pulmonary lymphatic disease presenting as interstitial lung disease in adulthood: report of 3 cases. Am J Surg Pathol 2012;36:1548-54. [Crossref] [PubMed]
- Havlik DM, Massie LW, Williams WL, et al. Pulmonary capillary hemangiomatosis-like foci. An autopsy study of 8 cases. Am J Clin Pathol 2000;113:655-62. [Crossref] [PubMed]
- Fugo K, Matsuno Y, Okamoto K, et al. Solitary capillary hemangioma of the lung: report of 2 resected cases detected by high-resolution CT. Am J Surg Pathol 2006;30:750-3. [Crossref] [PubMed]
- Hashimoto H, Kurata A, Fujiwara M, et al. Solitary Pulmonary Capillary Hemangioma of Adult Cases: Clinicopathologic Characteristics as an Unrecognized Entity. Am J Surg Pathol 2016;40:1380-9. [Crossref] [PubMed]
- Hashimoto H, Yanagiya M, Suzuki Y, et al. A case of solitary pulmonary capillary hemangioma indicating true gross appearance. Pathol Int 2017;67:322-3. [Crossref] [PubMed]
- Giannini C, Rushing EJ, Hainfellner, et al. Solitary fibrous tumor/hemangiopericytoma. In: Louis DN, Ohgaki H, Wiestler OD, et al. editors. WHO Classification of Tumours of the Central Nervous System. Revised 4th ed. Lyon: International Agency for Research on Cancer, 2016:249-54.
- Rao N, Colby TV, Falconieri G, et al. Intrapulmonary solitary fibrous tumors: clinicopathologic and immunohistochemical study of 24 cases. Am J Surg Pathol 2013;37:155-66. [Crossref] [PubMed]
- Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390-5. [Crossref] [PubMed]
- Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 2014;38:552-9. [Crossref] [PubMed]
- Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 2013;45:131-2. [Crossref] [PubMed]
- Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180-5. [Crossref] [PubMed]
- Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol 2012;25:1298-306. [Crossref] [PubMed]
- Tapias LF, Mercier O, Ghigna MR, et al. Validation of a scoring system to predict recurrence of resected solitary fibrous tumors of the pleura. Chest 2015;147:216-23. [Crossref] [PubMed]
- Reisenauer JS, Mneimneh W, Jenkins S, et al. Comparison of Risk Stratification Models to Predict Recurrence and Survival in Pleuropulmonary Solitary Fibrous Tumor. J Thorac Oncol 2018;13:1349-62. [Crossref] [PubMed]
- Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509-20. [Crossref] [PubMed]
- Takeuchi K, Soda M, Togashi Y, et al. Pulmonary inflammatory myofibroblastic tumor expressing a novel fusion, PPFIBP1-ALK: reappraisal of anti-ALK immunohistochemistry as a tool for novel ALK fusion identification. Clin Cancer Res 2011;17:3341-8. [Crossref] [PubMed]
- Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 2010;363:1727-33. [Crossref] [PubMed]
- Panigrahi MK, Pradhan G, Sahoo N, et al. Primary pulmonary synovial sarcoma: A reappraisal. J Cancer Res Ther 2018;14:481-9. [Crossref] [PubMed]
- Zeren H, Moran CA, Suster S, et al. Primary pulmonary sarcomas with features of monophasic synovial sarcoma: a clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum Pathol 1995;26:474-80. [Crossref] [PubMed]
- Hartel PH, Fanburg-Smith JC, Frazier AA, et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol 2007;20:760-9. [Crossref] [PubMed]
- Lan T, Chen H, Xiong B, et al. Primary pleuropulmonary and mediastinal synovial sarcoma: a clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China. Diagn Pathol 2016;11:62. [Crossref] [PubMed]
- Lino-Silva LS, Flores-Gutiérrez JP, Vilches-Cisneros N, et al. TLE1 is expressed in the majority of primary pleuropulmonary synovial sarcomas. Virchows Arch 2011;459:615-21. [Crossref] [PubMed]
- Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res 2002;62:135-40. [PubMed]
- Kawaguchi S, Tsukahara T, Ida K, et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: a study from the Japanese Musculoskeletal Oncology Group. Cancer Sci 2012;103:1625-30. [Crossref] [PubMed]
- Thway K, Nicholson AG, Lawson K, et al. Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion: a new tumor entity. Am J Surg Pathol 2011;35:1722-32. [Crossref] [PubMed]
- Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol 2012;36:e1-11. [Crossref] [PubMed]
- Kao YC, Sung YS, Zhang L, et al. EWSR1 Fusions With CREB Family Transcription Factors Define a Novel Myxoid Mesenchymal Tumor With Predilection for Intracranial Location. Am J Surg Pathol 2017;41:482-90. [Crossref] [PubMed]
- Skálová A, Weinreb I, Hyrcza M, et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol 2015;39:338-48. [Crossref] [PubMed]
- Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer 2010;49:1114-24. [Crossref] [PubMed]
- Dal Cin P, Kools P, De Jonge I, et al. Rearrangement of 12q14-15 in pulmonary chondroid hamartoma. Genes Chromosomes Cancer 1993;8:131-3. [Crossref] [PubMed]
- Kazmierczak B, Rosigkeit J, Wanschura S. HMGI-C rearrangements as the molecular basis for the majority of pulmonary chondroid hamartomas: a survey of 30 tumors. Oncogene 1996;12:515-21. [PubMed]
- Schoenmakers EF, Wanschura S, Mols R, et al. Recurrent rearrangements in the high mobility group protein gene, HMGI-C, in benign mesenchymal tumours. Nat Genet 1995;10:436-44. [Crossref] [PubMed]
- Dahlén A, Mertens F, Rydholm A, et al. Fusion, disruption, and expression of HMGA2 in bone and soft tissue chondromas. Mod Pathol 2003;16:1132-40. [Crossref] [PubMed]
- Panagopoulos I, Bjerkehagen B, Gorunova L, et al. Rearrangement of chromosome bands 12q14~15 causing HMGA2-SOX5 gene fusion and HMGA2 expression in extraskeletal osteochondroma. Oncol Rep 2015;34:577-84. [Crossref] [PubMed]
- Rodriguez FJ, Aubry MC, Tazelaar HD, et al. Pulmonary chondroma: a tumor associated with Carney triad and different from pulmonary hamartoma. Am J Surg Pathol 2007;31:1844-53. [Crossref] [PubMed]
- Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med 2009;266:43-52. [Crossref] [PubMed]
- Deavers M, Guinee D, Koss MN, et al. Granular cell tumors of the lung. Clinicopathologic study of 20 cases. Am J Surg Pathol 1995;19:627-35. [Crossref] [PubMed]
- Rekhi B, Jambhekar NA. Morphologic spectrum, immunohistochemical analysis, and clinical features of a series of granular cell tumors of soft tissues: a study from a tertiary referral cancer center. Ann Diagn Pathol 2010;14:162-7. [Crossref] [PubMed]
- Filie AC, Lage JM, Azumi N. Immunoreactivity of S100 protein, alpha-1-antitrypsin, and CD68 in adult and congenital granular cell tumors. Mod Pathol 1996;9:888-92. [PubMed]
- Fanburg-Smith JC, Meis-Kindblom JM, Fante R, et al. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol 1998;22:779-94. [Crossref] [PubMed]
- Di Tommaso L, Magrini E, Consales A, et al. Malignant granular cell tumor of the lateral femoral cutaneous nerve: report of a case with cytogenetic analysis. Hum Pathol 2002;33:1237-40. [Crossref] [PubMed]
- Papachristou DJ, Palekar A, Surti U, et al. Malignant granular cell tumor of the ulnar nerve with novel cytogenetic and molecular genetic findings. Cancer Genet Cytogenet 2009;191:46-50. [Crossref] [PubMed]
- Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience from 1980 to 1993. Ann Intern Med 1996;124:407-13. [Crossref] [PubMed]
- Gillmore JD, Hawkins PN. Amyloidosis and the respiratory tract. Thorax 1999;54:444-51. [Crossref] [PubMed]
- Shiue ST, McNally DP. Pulmonary hypertension from prominent vascular involvement in diffuse amyloidosis. Arch Intern Med 1988;148:687-9. [Crossref] [PubMed]
- Hashimoto H, Kurata A, Mizuno H, et al. Pulmonary arterial hypertension due to pulmonary vascular amyloid deposition in a patient with multiple myeloma. Int J Clin Exp Pathol 2015;8:15391-5. [PubMed]
- Grogg KL, Aubry MC, Vrana JA, et al. Nodular pulmonary amyloidosis is characterized by localized immunoglobulin deposition and is frequently associated with an indolent B-cell lymphoproliferative disorder. Am J Surg Pathol 2013;37:406-12. [Crossref] [PubMed]
- Kijner CH, Yousem SA. Systemic light chain deposition disease presenting as multiple pulmonary nodules. A case report and review of the literature. Am J Surg Pathol 1988;12:405-13. [Crossref] [PubMed]
- Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012;366:539-51. [Crossref] [PubMed]
- Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;22:21-30. [Crossref] [PubMed]
- Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181-92. [Crossref] [PubMed]
- Zen Y, Inoue D, Kitao A, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol 2009;33:1886-93. [Crossref] [PubMed]
- Nagashima K, Sano I, Kobayashi T, et al. IgG4-related Lung Pseudotumor and Pleural Inflammation with Autoimmune Hepatitis. Intern Med 2018;57:43-8. [Crossref] [PubMed]
- Hashimoto H, Kurata A, Kuroda M, et al. Improvement of IgG4-related Disease with a Supplemental Steroid. Intern Med 2015;54:2949-50. [Crossref] [PubMed]
- Park JY, Sung CO, Jang SJ, et al. Pulmonary metastatic nodules of uterine low-grade endometrial stromal sarcoma: histopathological and immunohistochemical analysis of 10 cases. Histopathology 2013;63:833-40. [Crossref] [PubMed]
- Usui G, Hashimoto H, Matsumoto J, et al. Pulmonary Metastasis of Undifferentiated Uterine Sarcoma Forming Fluid-Containing Cyst. Int J Surg Pathol 2018;26:180-4. [Crossref] [PubMed]
- Aubry MC, Myers JL, Colby TV, et al. Endometrial stromal sarcoma metastatic to the lung: a detailed analysis of 16 patients. Am J Surg Pathol 2002;26:440-9. [Crossref] [PubMed]
- Traweek T, Rotter AJ, Swartz W, et al. Cystic pulmonary metastatic sarcoma. Cancer 1990;65:1805-11. [Crossref] [PubMed]
- Itoh T, Mochizuki M, Kumazaki S, et al. Cystic pulmonary metastases of endometrial stromal sarcoma of the uterus, mimicking lymphangiomyomatosis: a case report with immunohistochemistry of HMB45. Pathol Int 1997;47:725-9. [Crossref] [PubMed]
- Inamura K. Diagnostic and Therapeutic Potential of MicroRNAs in Lung Cancer. Cancers (Basel) 2017;9:E49. [Crossref] [PubMed]
- Inamura K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front Oncol 2017;7:193. [Crossref] [PubMed]
- Inamura K. Update on Immunohistochemistry for the Diagnosis of Lung Cancer. Cancers (Basel) 2018;10:E72. [Crossref] [PubMed]


