The evolution of fast track protocols after oesophagectomy
Introduction
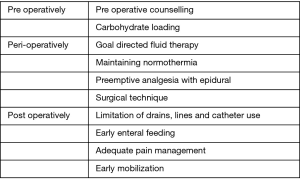
Fast track is a multimodal approach aimed at reducing the surgical stress response and improving postoperative recovery and return of functional status. Fast track protocols involve key elements aimed at optimization of crucial components in pre-, peri- and post-operative period (Figure 1).
The concept of fast track post-operative protocols for surgery was initially introduced by Kehlet in 1997 in which he described the risk factors associated with postoperative morbidity and duration of hospital stay (1). This led to the introduction of this multimodality system in colonic surgery in 1999. Several studies have shown improvements in clinical outcomes with the utilisation of fast track protocols in colonic surgery. It has been directly linked to decreasing length of stay (2,3) and reductions in the incidence and severity of postoperative complications (4,5). This led the ERAS (enhanced recovery after surgery) study group to publish a consensus statement in 2005 regarding a unified protocol for colonic surgery. Since then enhanced recovery has been applied in several subspecialties and guidelines have been published for colorectal surgery (6), gastrectomy (7), bariatric surgery (8), liver surgery (9) and gynaecologic oncology (10,11).
Surgical resection is the mainstay of treatment for locoregional oesophageal cancer (12) and is associated with high levels of mortality (30-day 2.4% and 90-day of 4.5%) and morbidity rate varying between 40–80% (13). The implementation of fast track protocols after oesophagectomy has been limited due to the high complexity of the procedure and the huge technical variations involved. Further some aspects are highly controversial for example one of main key components of ERAS is early feeding, which contradicts the traditional surgical concerns regarding early feeding leading to anastomotic leak. Fast track in the setting of oesophageal surgery was first introduced in 2004 (14), and since then several studies have investigated the effect of ERAS in this patient cohort. There are varieties in the ERAS protocols used and the ERAS society has recently published recommendations regarding fast track with oesophagectomy in the aim of a standardising the protocol for oesophagectomy which can be routinely applied and audited to improve patients’ outcomes (15).
The objective of this present review is to identify from the published literature, the evolution of fast track protocols for oesophagectomy over time and the changes in the key components that have led to a measurable improvement in postoperative outcomes. A systematic literature search was performed up until September 2018 using MEDLINE, Embase, Web of Science and the Cochrane Library databases. Figure 2 shows the PRISMA chart of the literature search.
Evolution of fast track protocols in oesophagectomy
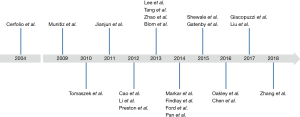
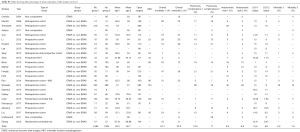
Both Comparative and non-comparative Cohort studies and randomised trials investigating the effect of ERAS with a clearly documented fast track pathway were included. Articles assessing the effect of one component of fast track were excluded from this section. A total of twenty-two publications were included in the analysis of ERAS pathway evolution. Of these, seven prospective cohort studies (16-22), eleven retrospective cohort studies (23-33), 1 study that used a combination of prospective and retrospective methods (34), two randomized control trials (35,36) and three non-comparative studies (14,37,38) were identified. Table S1 shows the key components in fast track protocol and the variation in the protocol used regarding those components. Figure 3 showing the timeline of the studies included in the fast track protocol development. The primary outcome measure was the length of hospital stay (defined at the time from surgery to discharge from hospital). Secondary outcome measures in-hospital mortality and postoperative complications, specifically anastomotic leak, and pulmonary complications (including pneumonia, persistent pneumothorax, and acute respiratory distress syndrome).

Full table
Perioperative protocols
No specific guidelines were followed regarding fluid management in the majority of the studies. In general all seven studies incorporating goal directed fluid management in their protocol, aimed at avoidance of fluid overload. In 2009 Munitiz et al. (23) aimed for a negative fluid balance four days postoperatively, whilst a 2015 cohort study targeted at an obtainment of an even balance without applying a restricted regimen (20). A 2017 study combining both pro- and retrospective study design defined a protocol with a negative fluid balance in the first days postoperatively and obtainment of an even balance on subsequent days (32). Most studies compared fast track protocols in patients undergoing open oesophagectomy. The first study incorporating patients treated with minimally invasive oesophagectomy (MIO) in the fast track protocol was by Li et al. Minimally invasive oesophagectomy was not integrated as an element of the fast track protocol as patients treated with MIO were included in both fast track and conventional care group (16).
A 2014 prospective cohort study made a comparison between patients undergoing open and laparoscopically assisted Ivor Lewis oesophagectomy. Fast track protocol was applied for both surgical approaches. However, the conventional group not following fast track consisted only of patients receiving open surgery. A significant reduction in the median postoperative length of hospital stay was achieved in the fast track group (10 days) in comparison with the conventional group (13 days). Nevertheless, no difference could be demonstrated between both surgical treatments within the fast track group. The authors could therefore not conclude that the reduction in length of hospital stay in the fast track group could be based on the surgical technique. (19) The only study assessing fast track in patients treated with MIO only was conducted by Pan et al. (28).
Postoperative management
The majority of the studies implemented immediate extubation in their fast track protocol. This was not applied in the first fast track study following oesophagectomy in 2004, yet intensive care unit (ICU) stay could also be avoided in the majority of these patients. Recent studies aimed at immediate extubation following surgery.
Since fast track protocol first used, there appears to be uniformity in the epidural removal within first five days and early mobilization. Additionally, no noticeable difference could be observed in the protocols regarding early chest tube removal. However, feeding in terms of early oral intake or jejunostomy feeding showed variation over the years, but most of the recent studies showed discharge on jejunostomy feeding. There also appears to be less use of gastrografin to assess the anastomosis and nasogastric tube removal.
Outcome measures
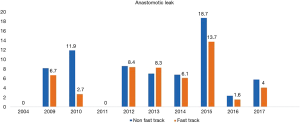
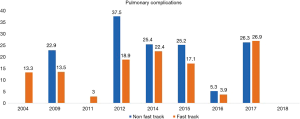
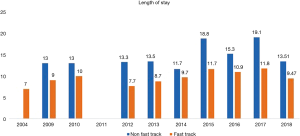
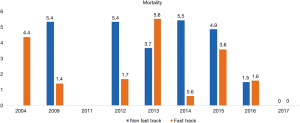
A total 22 publications were included in this section. Table S1 showing the studies included and a summary of the outcomes of interest. Overall, fast track had positive effect on patient outcomes. Anastomotic leak rate (Figure 4) was persistently lower in the ERAS group in comparison to the non-ERAS. Higher rate of anastomotic leaks in 2015 in comparison to other fast track studies could be explained due inclusion of clinically non-significant leaks in the study by Shewale et al. (29). Reduction in pulmonary complications and length of stay in the ERAS group (Figures 5 and 6). Also, the rate of anastomotic leak and pneumonia varied across the years which may reflect variation in the criteria used to define those outcomes. Mean length of stay did not exceed 12 days in the fast track groups, whilst this could extend up to a mean length of stay of 19 days in the conventional care groups. The mortality rate followed the same pattern and fast track led to reduction in mortality rate in comparison to traditional care (Figure 7).
Evolution of the individual fast track components
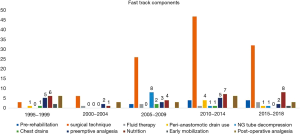
A total of 218 studies included were those investigating specific ERAS component, pre-rehabilitation, Surgical technique, Fluid therapy, preemptive analgesia, Peri-anastomotic drain use, NG tube decompression, chest drains, nutrition, early mobilisation and Post-operative Analgesia. Articles included in the fast track protocol evolution mentioned above were excluded in this section. The aim is to assess the frequency that have described that particular component, taking the year of publication into account. This will allow an overview understanding of the evolution of the importance of each component and its impact on recovery. Figure 8 shows the fast track components evolution, highlighting the aspects that has been mostly investigated have been surgical technique changes in the field of minimally invasive oesophagectomy as well as nutritional development. This goes hand in hand with the variation in practice that has been seen in Table S2.
Full table
Discussion
The evolution of fast track protocols for oesophagectomy demonstrates a continuous commitment to evaluation of service that has used more commonly in the recent years. This can be explained by studies utilising fast track guidelines adapted from other current approved protocols approved by ERAS study group and not specifically designed to be address the unique nature of the care needed with oesophageal resection; given it is the only general surgical operation which has a thoracic component.
Restrictive perioperative fluid therapy showed a reduction in complications after colonic surgery (39). A retrospective cohort study examined fluid management after oesophagectomy and found a strong correlation between postoperative fluid overload and increased postoperative morbidity (40). Goal directed fluid therapy has been reported in less than half of the studies utilising fast track protocols in the context of oesophagectomy. Goal directed fluid therapy to avoid hypervolemia is advocated, as fluid overload has been shown to be associated with higher rates of anastomotic leak, pneumonia (41).
Immediate postoperative extubation demonstrated its beneficial effect in other types of surgery regarding the length of ICU stay (42,43). However, no difference was found for the fast track post-oesophagectomy studies not implementing this in their protocol. Early mobilisation was used in almost all the studies with fast track in oesophagectomy. It has been shown early postoperative mobilization improves cardiovascular and pulmonary functioning and reduces the risk of thromboembolic complications (1). It also has longer term benefits after discharge of showed significant improvement in such parameters as fatigue, sleep, return to leisure activity and activities of daily living (44). Taniguchi et al. (45) found that goal directed fluid therapy enhanced postoperative gastrointestinal recovery and mobilisation, as well as postoperative nutritional status and protein synthesis. The program did not affect either postoperative LOS or the incidence of complications.
Almost all studies commonly placed NG tube during the surgery and most are removed within 5 days with or without gastrografin study prior to removal. Only three studies did not use NG tube routinely (25,27,35). There is current evidence that NG tubes can increase the risk of postoperative respiratory tract infection (46). In addition, it was shown that NG tube post oesophagectomy led to significant higher rate of anastomotic leak as well as leading to longer length of stay and an increase in pulmonary complications (47).
Feeding has been a source of discrepancy between studies, this is due to the thought that early feeding can lead to anastomotic leak and aspiration. Martos-Benítez et al. (48) showed that early enteral feeding in gastrointestinal surgery led to a significant reduction in major complications, respiratory complications, and gastrointestinal complications specifically anastomotic leak. There was also reduction in length of hospital stay. Specifically, in relation to oesophageal surgery, Cao et al. (25) showed no significant differences in anastomotic leak rate in their study with initiation of jejunostomy feed on day 1 as well as oral intake on day 4. Early enteral feeding is an important part of any fast track program. Enteral feeding via feeding tube was shown to reduce anastomotic leak, wound and other infections, pneumonia, and mortality. There is also an associated reduction of length of stay (49).
Chest tube is believed to allow for early detection and management of anastomotic leakage. Cao et al. (25) showed that anastomotic leak rate was not significantly different between early removal of chest tube in fast track and late removal. However, early removal of chest tube was one of several factors that shortens length of hospital stay.
Postoperative pain is a major factor in the recovery of patients after esophageal surgery and adequate pain control is believed to decrease cardiopulmonary complications, length of hospital-stay, and mortality. Epidural analgesia has been the choice of analgesia post oesophagectomy due to the reduction in cardiopulmonary complications and length of stay. However, anastomotic leak was not statistically different with the use of epidural (50). Later studies, Li et al. (51) in a cohort of 587 patients have shown that the use of epidural in oesophagectomy has led to significantly reduced rate of pneumonia from 32% to 19.7% and anastomotic lean 23.0% to 14.0%. Michelet et al. (52) have also shown that that epidural analgesia is associated with a decreased incidence of anastomotic leak.
The surgical technique in the form of minimally invasive oesophagectomy has been a major advancement in the field. Minimally invasive oesophagectomy was first introduced in 1992 (53). There has been two randomised controlled trials (54,55) which have revealed the feasibility of a minimally invasive technique with evidence of short term benefits as well as a comparable lymph node yield in comparison to open surgery which mounts to good oncological resection. Despite this the majority of resections carried out through an open technique (56), and there are currently ongoing trials to delineate the advantages of minimally invasive versus open such The ROMIO (Randomized Oesophagectomy: Minimally Invasive or Open) trial (57), The ROBOT trial (58). Recently, a propensity-matched population-based study from the Dutch Esophagectomy revealed that the pulmonary complication rate and mortality for minimally invasive surgery were similar to open technique. However, anastomotic leaks and the need for re-interventions were more significant in minimally invasive surgery. The length of stay was shorter in minimally invasive group (59). This will have a further positive effect on enhancing the recovery of such complex patients. To date no studies have been conducted comparing MIO as an element of fast track program to conventional care with an open surgical approach. Early studies regarding fast track after oesophagectomy have been assessing protocols for open surgery alone. The first study to incorporate MIO was done by Li et al. in 2012 (16). Later studies also made a comparison of fast track programs versus conventional care in which patients treated with MIO were included in both study groups. Therefore, impact of a minimally invasive approach as part of a fast track protocol on recovery could not be assessed.
In summary, Fast track protocols is oesophagectomy shows variations in practice due to the complexity of the procedure. Fast track has been shown to reduce hospital stay and morbidity following oesophagectomy. It has been advocated that early mobilisation, early enteral feeding, early removal of chest tube, limiting the use of nasogastric decompression and optimizing the use of epidural anaesthesia or analgesia facilitates early discharge of patients. Recommendations for standardised pathway post oesophagectomy has been recently published by ERAS study group, this will allow the outcomes to be assessed in a unified manner as well as allowing the auditing of fats track pathway.
Acknowledgements
Mr. Sheraz Markar is funded by the National Institute of Health Research NIHR.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Martin TD, Lorenz T, Ferraro J, et al. 2016Newly implemented enhanced recovery pathway positively impacts hospital length of stay. Surg Endosc 2016;30:4019-28. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Schwenk W, et al. 2013 Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg 2013;37:259-84. [Crossref] [PubMed]
- Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011.CD007635. [PubMed]
- Pecorelli N, Hershorn O, Baldini G, et al. Impact of adherence to care pathway interventions on recovery following bowel resection within an established enhanced recovery pro- gram. Surg Endosc 2017;31:1760-71. [Crossref] [PubMed]
- Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961-9. [Crossref] [PubMed]
- Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Br J Surg 2014;101:1209-29. [Crossref] [PubMed]
- Thorell A, MacCormick AD, Awad S, et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 2016;40:2065-83. [Crossref] [PubMed]
- Melloul E, Hubner M, Scott M, et al. Guidelines for perioperative care for liver surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 2016;40:2425-40. [Crossref] [PubMed]
- Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recom- mendations, part I. Gynecol Oncol 2016;140:313-22. [Crossref] [PubMed]
- Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations, part II. Gynecol Oncol 2016;140:323-32. [Crossref] [PubMed]
- Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol 2003;4:481-8. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complica- tions associated with esophagectomy: Esophagectomy Compli- cations Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Bass CS, et al. Fast racking adter Ivor Lewis esophagectomy. Chest 2004;126:1187-94. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Li C, Ferri LE, Mulder DS, et al. An enhanced recovery pathway decreases duration of stay after esophagectomy. Surgery 2012;152:606-14. [Crossref] [PubMed]
- Markar SR, Schmidt H, Kunz S, et al. Evolution of standardized clinical pathways: refining multidisciplinary care and process to improve outcomes of the surgical treatment of esophageal cancer. J Gastrointest Surg 2014;18:1238-46. [Crossref] [PubMed]
- Findlay JM, Tustian E, Millo J, et al. The effect of formalizing enhanced recovery after esophagectomy with a protocol. Dis Esophagus 2015;28:567-73. [Crossref] [PubMed]
- Ford SJ, Adams D, Dudnikov S, et al. The implementation and effectiveness of an enhanced recovery programme after oesophago-gastrectomy: a prospective cohort study. Int J Surg 2014;12:320-4. [Crossref] [PubMed]
- Blom RL, van Heijl M, Bemelman WA, et al. Initial experiences of an enhanced recovery protocol in esophageal surgery. World J Surg 2013;37:2372-8. [Crossref] [PubMed]
- Akiyama Y, Iwaya T, Endo F, et al. Effectiveness of intervention with a perioperative multidisciplinary support team for radical esophagectomy. Support Care Cancer 2017;25:3733-9. [Crossref] [PubMed]
- Zhao G, Cao S, Cui J. Fast-track surgery improves postoperative clinical recovery and reduces postoperative insulin resistance after esophagectomy for esophageal cancer. Support Care Cancer 2014;22:351-8. [Crossref] [PubMed]
- Munitiz V, Martinez-de-Haro LF, Ortiz A, et al. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br J Surg 2010;97:714-8. [Crossref] [PubMed]
- Tomaszek SC, Cassivi SD, Allen MS, et al. An alternative postoperative pathway reduces length of hospitalisation following oesophagectomy. Eur J Cardiothorac Surg 2010;37:807-13. [Crossref] [PubMed]
- Cao S, Zhao G, Gui J, et al. Fast-track rehabilitation program and conventional care after esophagectomy: a retrospective controlled cohort study. Support Care Cancer 2013;21:707-14. [Crossref] [PubMed]
- Preston SR, Markar SR, Baker CR, et al. Impact of a multidisciplinary standardized clinical pathway on perioperative outcomes in patients with oesophageal cancer. Br J Surg 2013;100:105-12. [Crossref] [PubMed]
- Lee L, Li C, Robert N, et al. Economic impact of an enhanced recovery pathway for oesophagectomy. Br J Surg 2013;100:1326-34. [Crossref] [PubMed]
- Pan H, Hu X, Yu Z, et al. Use of a fast-track surgery protocol on patients undergoing minimally invasive oesophagectomy: preliminary results. Interact Cardiovasc Thorac Surg 2014;19:441-7. [Crossref] [PubMed]
- Shewale JB, Correa AM, Baker CM, et al. Impact of a Fast-track Esophagectomy Protocol on Esophageal Cancer Patient Outcomes and Hospital Charges. Ann Surg 2015;261:1114-23. [Crossref] [PubMed]
- Gatenby PA, Shaw C, Hine C, et al. Retrospective cohort study of an enhanced recovery programme in oesophageal and gastric cancer surgery. Ann R Coll Surg Engl 2015;97:502-7. [Crossref] [PubMed]
- Oakley B, Lamb C, Vohra R, et al. Achieving long term survival in oesophagectomy patients aged over 75. Ann Med Surg (Lond) 2016;9:15-21. [Crossref] [PubMed]
- Giacopuzzi S, Weindelmayer J, Treppiedi E, et al. Enhanced recovery after surgery protocol in patients undergoing esophagectomy for cancer: a single center experience. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Liu YW, Yan FW, Tsai DL, et al. Expedite recovery from esophagectomy and reconstruction for esophageal squamous cell carcinoma after perioperative management protocol reinvention. J Thorac Dis 2017;9:2029-37. [Crossref] [PubMed]
- Tang J, Humes DJ, Gemmil E, et al. Reduction in length of stay for patients undergoing oesophageal and gastric resections with implementation of enhanced recovery packages. Ann R Coll Surg Engl 2013;95:323-8. [Crossref] [PubMed]
- Chen L, Sun L, Lang Y, et al. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016;16:449. [Crossref] [PubMed]
- Zhang Z, Zong L, Xu B, et al. Observation of clinical efficacy of application of enhanced recovery after surgery in perioperative period on esophageal carcinoma patients. J BUON 2018;23:150-6. [Crossref] [PubMed]
- Underwood TJ, Noble F, Madhusudan N, et al. The Development, Application and Analysis of an Enhanced Recovery Programme for Major Oesophagogastric Resection. J Gastrointest Surg 2017;21:614-21. [Crossref] [PubMed]
- Jianjun Q, Yin L, Wenqun X, et al. Fast track program for esophagectomy patients. Thorac Cancer 2012;3:55-9. [Crossref] [PubMed]
- Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomized controlled trial. Lancet 2002;359:1812-8. [Crossref] [PubMed]
- Glatz T, Kulemann B, Marjanovic G, et al. Postoperative fluid overload is a risk factor for adverse surgical outcome in patients undergoing esophagectomy for esophageal cancer: a retrospective study in 335 patients. BMC Surg 2017;17:6. [Crossref] [PubMed]
- Chappell D, Jacob M. Influence of non-ventilatory options on postoperative outcome. Best Pract Res Clin Anaesthesiol 2010;24:267-81. [Crossref] [PubMed]
- Mandell MS, Lezotte D, Kam I, et al. Reduced use of intensive care after liver transplantation: Influence of early extubation. Liver Transpl 2002;8:676-81. [Crossref] [PubMed]
- Chamchad D, Horrow JC, Nachamchik L, et al. The impact of immediate extubation in the operating room after cardiac surgery on intensive care and hospital lengths of stay. J Cardiothorac Vasc Anesth 2010;24:780-4. [Crossref] [PubMed]
- Hjort Jakobsen D, Sonne E, Basse L, et al. Convalescence after colonic resection with fast-track versus conventional care. Scand J Surg 2004;93:24-8. [Crossref] [PubMed]
- Taniguchi H, Sasaki T, Fujita H, et al. Effects of goal-directed fluid therapy on enhanced postoperative recovery: An interventional comparative observational study with a historical control group on oesophagectomy combined with ERAS program. Clin Nutr ESPEN 2018;23:184-93. [Crossref] [PubMed]
- Daryaei P, Vaghef Davari F, Mir M, Harirchi I, Salmasian H. Omission of nasogastric tube application in postoperative care of esophagectomy. World J Surg 2009;33:773-7. [Crossref] [PubMed]
- Nguyen NT, Slone J, Woolridge J, et al. Minimally invasive oesophagostomy without the use of postoperative nasogastric tube decompression. Am Surg 2009;75:929-31. [PubMed]
- Martos-Benítez FD, Gutiérrez-Noyola A, Soto-García A, et al. Program of gastrointestinal rehabilitation and early postoperative enteral nutrition: a prospective study. Updates Surg 2018;70:105-12. [Crossref] [PubMed]
- Lewis SJ, Egger M, Sylvester PA, et al. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ 2001;323:773-6. [Crossref] [PubMed]
- Tsui SL, Law S, Fok M, et al. Postoperative analgesia reduces mortality and morbidity after esophagectomy. Am J Surg 1997;173:472-8. [Crossref] [PubMed]
- Li W, Li Y, Huang Q, et al. Short and Long-Term Outcomes of Epidural or Intravenous Analgesia after Esophagectomy: A Propensity-Matched Cohort Study. PLoS One 2016;11:e0154380. [Crossref] [PubMed]
- Michelet P, D'Journo XB, Roch A, et al. Perioperative risk factors for anastomotic leak- age after esophagectomy: influence of thoracic epidural analgesia. Chest 2005;128:3461-6. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mariette V, Meunier B, Pezet D, et al. Hybrid minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicenter, open-label, randomized phase III controlled trial, the MIRO trial. J Clin Oncol 2015;33:5. [Crossref]
- Allum WH, Bonavina L, Cassivi SD, et al. Surgical treatment for esophageal cancers. Ann N Y Acad Sci 2014;1325:242-68. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- Avery KN, Metcalfe C, Berrisford R, et al. The feasibility of a randomized controlled trial of esophagectomy for esophageal cancer—the ROMIO (Randomized Oesophagectomy: Minimally Invasive or Open) study: protocol for a randomized controlled trial. Trials 2014;15:200. [Crossref] [PubMed]
- Seesing MFJ, Gisbertz SS, Goense L, et al. A propensity score matched analysis of open versus minimally invasive transthoracic esophagectomy in the Netherlands. Ann Surg 2017;266:839-46. [Crossref] [PubMed]