
Gross handling of pulmonary resection specimen: maintaining the 3-dimensional orientation
Introduction
The literature in pathology about handling of surgical specimens is underrepresented. Accurate precise staging, adequate microscopical examination and predictive testing of a surgical specimen, are fully dependent on adequate gross handling. Proper handling of the surgical specimens requires a thorough appreciation of staging system and sufficient clinical and radiological information.
Guidelines for gross handling of surgical specimens with lung tumors are marginally defined in the literature: the College of American Physicians (CAP) formulates “required elements” for synoptic reporting regarding gross handling of pulmonary resection specimen, mainly focusing on pathology staging, but does not include specified grossing requirements (1). There are a couple of valuable publications on the topic of challenges in pathologic staging, focusing mainly on the general issues of the TNM staging, precision of the pathological tumor stage and microscopic issues of pleural invasion, tumor distance to carina and staging of synchronous tumors (2,3). According to Flieder, the pathological stage is as good as the patient’s surgeon and surgeon’s pathologist (3).
In this paper we report a 3-dimensional (3D) approach of gross handling of pulmonary resection specimens used in VU University Medical Center in Amsterdam, allowing accurate correlation with the pre-surgical imaging.
Methods
The current practice of handling resection specimen in the Department of Pathology, VU University Medical Center in Amsterdam involves several crucial steps.
Interaction with surgeon for submission of pulmonary specimen
There are several types of pulmonary resections in lung pathology: lobectomy, bilobectomy, pneumectomy, lobectomy with parts of thoracic wall (e.g., Pancoast), segmentectomy, wedge resection and sleeve resection. The required information includes: (I) site of specimen, i.e., resected lobe(s) and in case of partial thoracic wall resection, additional information on the resected ribs or vertebra; (II) type of surgery, including pneumonectomy, (sleeve) lobectomy, segmentectomy, and wedge resection; (III) number of tumors if multiple; (IV) in case of extended resections, additional information on resected thoracic wall, vertebra, mediastinal organs, diaphragm, parietal pleura, additional wedge excision from adjacent lobe due to adhesion etc. should be included; (V) information about pretreatment including chemoradiation and immunotherapy.
In the case of the frozen section for diagnosis and/or resection margin, any useful information on orientation of the specimen to the definite resection margins, which are to be examined, should be included. In the case of a resected bronchial slice, designation of the margin of the largest distance from the hilum of the patient (“cold” side), is important. A mark on the bronchial ring can be a suture through the cold side. Eventual epithelial damage on the cold side for frozen section analysis is less relevant, as the warm side needs to be examined microscopically on frozen section: i.e., the actual mucosal resection margin closest to the hilum. Further processing of a frozen section specimen is discussed in more detail in a separate section below.
Delivery of the pulmonary resection specimen to a pathology ward should be done as quickly as possible, in order to reduce the cold ischemia time to a minimum. This is particularly important in pulmonary resections, as autolysis decreases the quality of immunohistochemistry (4) and molecular analyses (5), which are increasingly important for the predictive testing for treatment of the patient in case of relapse. If quick transfer to the pathology ward is not possible, e.g., in a case of emergency surgery in the night, the specimen should be kept refrigerated on a temperature of 2–8 °C and processed the next day as soon as possible.
Handling of a fresh lung resection specimen
Firstly, the handling of a standard lobectomy will be described (Table 1). The pathologist begins with orientation of the specimen, maintaining the 3D anatomical orientation (in axial, sagittal and coronal planes). Specimen is photographed from the medial and lateral direction delineating the 3D orientation (Figure 1) on the images.

Full table
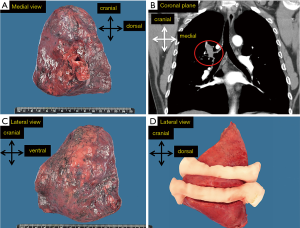
Description of the fresh specimen includes the surface of the pleura (tumor ingrowth, adhesion to parietal pleura which might also be resected, or pleural deposits and hilar resection surface. In case of tumor close to surface of the specimen, this side may be inked to facilitate microscopic examination. If there is an easily visible intrabronchial tumor, the distance to the bronchial resection margin should be measured. This is particularly important for the staging of small tumors (T1 <2 cm) as distance of <2 cm to carina indicates higher tumor grade (T2) (6).
Next, the bronchial resection margin is sampled in the fresh specimen and put in the cassette with the patient side down (“warm” side). This side will be cut first in paraffine embedded block and represents microscopically the true resection margin. This is particularly important in resections with a centrally located tumor where the bronchial resection margin to the tumor is small and inappropriate handling might lead to false positive margins. Short bronchial resection margins are commonly taken in carcinoid tumor resections in order to spare the resection of additional lobes.
Tumor is cut in axial direction in subtotal cuts so that the slices are still attached to the rest of the specimen, maintaining the orientation of the slices. The slices should not be thicker than 1.5 cm. The slices are held apart during fixation with thick bandage mesh in the cut (Figure 1D). Cutting the fresh tumor enables the sampling for the purpose of cancer-biobanking. Moreover, the bandage enables penetrance of the fixative in the slices from each side, in order to reach fixation of the entire tumor within 24 hours (with a neutral buffered 10% formalin penetrance rate of 2–36 mm per hour at room temperature, in collapsed lung tissue in our experience even less, around 0.2 mm per hour) (7). As it has been proven that loose tissue fragments are caused by gross cutting, the knife is rinsed and quickly wiped after each slice that contains tumor. So called ‘spread through airways’ (STAS) may be an artifact, and it is not currently taken into account in determination of resection margin status or field of radiotherapy (6,8).
Subsequently, preexistent (non-tumorous) lung parenchyma should be perfused with a large amount of neutral buffered formalin using a syringe, by injecting the formalin through pleural surface. Alternatively, perfusion of the lung is performed through the main or lobar bronchus. Afterwards the whole specimen is submerged in a large volume neutral buffered formalin.
In a pneumonectomy specimen, possible tumor extension in the interlobar fissures needs specific attention.
In a sleeve lobectomy specimen, two bronchial resection margins are identified and sampled separately. Recognition of the proximal and distal bronchial resection margin is possible by the smaller diameter of the distal compared to the central bronchus.
In a peripheral wedge resection or segment resection orientation can be challenging. Same principals are applied, namely enabling proper fixation of the tumor and peripheral lung tissue.
In tumors invading the chest wall (e.g., Pancoast tumor), the ‘en-bloc’ resection includes extrapulmonary structures directly invaded by tumor, usually ribs or part of vertebrae. During the fresh handling, the thoracic wall part of the specimen is removed from the lobectomy part. Subsequently, both cut surfaces are inked in same color by the pathologist to denote the artificial edges. Note that using the different ink color from the real resection margin, as mentioned above, will prevent misinterpretation during microscopic examination.
Bone requires after fixation extra time for decalcification, extending this part of the pathology reporting with additional few days.
Handling after formalin fixation
After 24 hours fixation the resection specimen is considered to be well fixed and handling is pursued. Specimens received on Friday are left to fixate over the weekend. Fixation longer than 24 hours does not influence the performance of most immunohistochemical markers used nowadays, due to improved antigen retrieval techniques (9). Hilar vascular resection margins are sampled. Hilar soft tissue resection margin may additionally be inked, if tumor is close to margin. To this end the formalin fixative on the surface may be wiped with paper and quickly dipped in 100% alcohol, which will evaporate. The dry surface is subsequently inked. This quick drying procedure prevents distribution of ink to a wider area than intended.
Metastases in hilar lymph nodes with extranodular tumor growth, might endanger the hilar soft tissue margin. Loose hilar lymph nodes are removed and cut in slices of 3 mm and then are completely embedded, preferably in one cassette. Mediastinal lymph nodes are always embedded separately, per lymph node station.
The specimen is further cut in slices along the same plane as in fresh situation, maintaining the three-dimensional orientation. Definite slices should not be thicker than 5 mm in order to ensure a thickness close to the resolution of the preoperative CT scan. Moreover, thin slices enable an easier evaluation of the presence of a solid component in tumors consisting predominantly of adenocarcinoma in situ component.
Slices are positioned in order of cutting, then numbered and a photograph is taken (Figure 2A). The tumor is described, including dimensions in centimeters, in three plains with precision 0.1 cm. Moreover, tumor localization, necrosis and fibrosis are noted. Distance to the hilar and bronchial resection margin is recorded. Relation to the pleura is described and if ingrowth is suspected, careful examination and proper sampling is prompted as it is one of the challenging microscopic issues in lung pathology (10). Sufficient sampling includes the relation of the tumor with the described structures, with a minimum of three tumor blocks. Tumors with a diameter ≤3 cm are completely embedded to ensure an adequate classification in case with an adenocarcinoma in situ component [adenocarcinoma in situ versus (minimally) invasive adenocarcinoma].
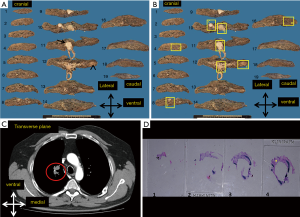
Any other changes of parenchyma need to be described and sampled, i.e., nodules, postobstruction pneumonia, emphysema. This may yield valuable information on the intrapulmonary metastases but also on fibrosis or infection and may offer a correlation with the preoperative imaging. At least two blocks of the preexistent lung parenchyma are sampled. This is particularly important in carcinoid tumors to evaluate the presence of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) (11). If DIPNECH is suspected, more extensive sampling of preexistent lung needs to be considered.
All the sampled tissue blocks are annotated on the photograph(s) with 3D orientation of the slices (Figure 2B). These pictures are also helpful to understand the gross handling, especially if another person than the one who handled the gross specimen will perform microscopic evaluation. Moreover, it allows supervision in a training situation and the correlation with the preoperative imaging (Figure 2C).
In peripheral wedge resections, resection margins are parenchymal margins, which are represented by the tissue at the staple line(s). The staples are cut from the specimen, and in our institution not further examined, if adjacent tissue is macroscopically free. Adjacent tissue is sampled for microscopic examination. If this penultimate staple margin section does not contain tumor, the margin is free (R0). However, if this contains tumor, an educated guess is reasonable, encompassing the amount of tumor compared to the other sections, and the estimation of the staple thickness (2 mm). If tumor is close to the staples, alternative approach is to remove a few staples from the staple line prior to cutting slices through tumor. Then a segment is cut perpendicular to the staple line exactly where the staples have been removed, containing the true resection margin and the tumor, which is submitted for microscopic examination.
Resection of the lung including parts of thoracic wall (so called ‘en-bloc resection’) requires three-dimensional orientation as well. Soft tissue margin of thoracic wall is inked. Thoracic wall, most commonly ribs, are sawed perpendicular to the ribs for practical purposes. After fixation slow decalcification with Kristensen solution (12) of approximately one week is indicated. These patients are commonly treated with induction chemoradiation therapy prior to resection in order to reduce the tumor size and increase the chance for surgical resection with free margins. Tumor should be sampled more extensively in order to evaluate the regression as tumors with complete or subtotal regression (<10% of tumor load) have favorable prognosis (13,14). If no microscopical tumor is found in the initially embedded tissue, more extensive sampling needs to be performed for possible detection of small clusters of vital tumor.
Handling of frozen tissue sections
In the case of a frozen tissue section of the bronchial resection margin, side closest to the patient is examined first, therefore placed upward on the frozen template (15). This side is often irregular. In case of an irregular resection surface, maximally 6 frozen sections are cut until a complete circumference is achieved, and are placed on microscopic slides and stained with H&E (Figure 2D). The bronchial resection margin is considered tumor-free, if the complete circumferential margin does not contain tumor. If needed, the sequential sections with incomplete circumference (up to 6 sections) may be evaluated to determine margin status. If only tumor cells are found in lymphatic vessels of the bronchial wall (but no direct invasion in the wall tissue), this will be reported, but not considered necessary for additional bronchial tissue procurement. The “R”, annotation of the resection margins in the TNM classification, is used as follows: R0 = free resection margins; R1 = microscopic margins not free; R2 = margins not free during gross pathology examination/surgery (6).
Discussion
Here we present a method for gross handling of pulmonary resection specimens maintaining the 3D orientation, which integrates surgical and radiological information with gross pathology, resulting in a precise pathological TNM staging of the specimens. The protocol has several advantages: (I) it enables microscopical evaluation of all the relevant parameters for determination of a pathological staging; (II) it results in feedback during the multidisciplinary oncology conference to the radiologist (16) and the surgeon; (III) as the pre-existing lung is perfused during fresh handling, the effect of collapse may be diminished; (IV) understanding of the grossing is possible due to the photographs of the specimen in different stages of the process; (V) quality of fixation of the specimen that may have an impact in predictive marker testing can be monitored.
Comparison of gross tumor size in radiology with pathology shows in protocols with perfusion fixation on average equal sizes (17), while without perfusion fixation the pathology size is lower than the radiology size (18-20).
The photographs made during the gross handling with 3D annotation will clarify the spatial orientation of the specimen compared to the preoperative CT imaging. The photographs are particularly valuable in an educational setting, where residents performed the gross handling and also in case a pathologist was not involved in gross handling and will be judging the microscopic slides. In addition, the visual feedback of the resection specimen during the multidisciplinary oncology conferences helps outlining areas with (microscopic) residual disease, and if present, aim in delineating the site(s) for post-operative radiotherapy, if needed.
Reducing the ex-vivo artifact of surgical collapse (21) by filling the alveolar spaces with formalin through the perfusion fixation step mimicks the ‘in vivo air’. This will reduce the chance of erroneously diagnosing collapsed adenocarcinoma in situ as papillary or acinar carcinoma (22).
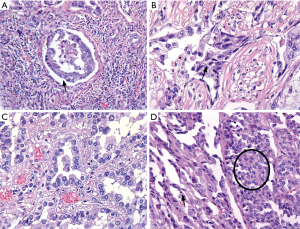
An underestimated issue in pathology is the influence of the delay in fixation time on the quality of the microscopic slides, immunohistochemistry and predictive biomarker testing. Delay of fixation or inadequate preparation of the specimen for the fixation will both result in cold ischemia. As the diffusion of formaldehyde fixation in uncut lungs may be slow (0.2 mm/hour in our experience), central parts of the lung may after 24 hours still be red (read: unfixed), if the specimen is inappropriately handled, cold ischemia results in morphological artifacts, like a gap artifact between tumor and stroma, loss of nuclear details, clumping of the nucleus and cytoplasm (23) (Figure 3). A loss of 30% to 50% of mitotic figures, because of delay in fixation by 2 to 6 hours has been reported in one study (24). This may influence diagnosis of carcinoid tumors. Moreover, quality of predictive immunohistochemistry (i.e., PD-L1) in lung pathology is an emerging issue. From the studies in breast cancer we know that some immunohistochemical stains are decreased due to delayed fixation (4,25). Studies of this issue in predictive lung pathology are pending. Even though molecular techniques nowadays are optimized for analysis of formalin fixed tissue with fragmented DNA and RNA, delayed fixation results in lysis of DNA and especially RNA by tissue D(-R)NAses (5,26). In short, uniform gross handling is one of the steps to reduce the variation in the preanalytical phase of tissue processing, influencing diagnostic pathology and predictive biomarker testing.
A disadvantage of this 3D handling protocol is that the processing may be time consuming. The presence of easily available photography at the grossing room will increase the efficiency.
In conclusion, we described a gross handling protocol that maintains the 3D orientation which facilitates precise staging, feedback during the multidisciplinary oncology conference and quality control of gross handling.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kelly Butnor AJ, Beth Beasley M, Cagle PT, et al. Protocol for the Examination of Specimens From Patients With Primary Non-Small Cell Carcinoma, Small Cell Carcinoma, or Carcinoid Tumor of the Lung Based on AJCC/UICC TNM, 7th edition. Available online: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution Folders/WebContent/pdf/cp-lung-16protocol-3400.pdf. Accessed May 16, 2018.
- Dacic S. Dilemmas in Lung Cancer Staging. Arch Pathol Lab Med 2012;136:1194-7. [Crossref] [PubMed]
- Flieder DB. Commonly encountered difficulties in pathologic staging of lung cancer. Arch Pathol Lab Med 2007;131:1016-26. [PubMed]
- Portier BP, Wang Z, Downs-Kelly E, et al. Delay to formalin fixation ‘cold ischemia time’: effect on ERBB2 detection by in-situ hybridization and immunohistochemistry. Mod Pathol 2013;26:1-9. [Crossref] [PubMed]
- van Maldegem F, de Wit M, Morsink F, et al. Effects of processing delay, formalin fixation, and immunohistochemistry on RNA Recovery From Formalin-fixed Paraffin-embedded Tissue Sections. Diagn Mol Pathol 2008;17:51-8. [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Helander KG. Kinetic studies of formaldehyde binding in tissue. Biotech Histochem 1994;69:177-9. [Crossref] [PubMed]
- Blaauwgeers H, Flieder D, Warth A, et al. A Prospective Study of Loose Tissue Fragments in Non–Small Cell Lung Cancer Resection Specimens An Alternative View to " Spread Through Air Spaces Am J Surg Pathol 2017;41:1226-30. [Crossref] [PubMed]
- von Wasielewski R, Werner M, Nolte M, et al. Effects of antigen retrieval by microwave heating in formalin-fixed tissue sections on a broad panel of antibodies. Histochemistry 1994;102:165-72. [Crossref] [PubMed]
- Travis WD, Brambilla E, Rami-Porta R, et al. Visceral Pleural Invasion: Pathologic Criteria and Use of Elastic Stains: Proposal for the 7th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2008;3:1384-90.
- Wirtschafter E, Walts AE, Liu ST, et al. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia of the Lung (DIPNECH): Current Best Evidence. Lung 2015;193:659-67. [Crossref] [PubMed]
- Kristensen HK. An Improved Method of Decalcification. Stain Technol 1948;23:151-4. [Crossref] [PubMed]
- Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer : morphology and prognosis. Chest 2001;120:1584-91. [Crossref] [PubMed]
- Blaauwgeers JL, Kappers I, Klomp HM, et al. Complete pathological response is predictive for clinical outcome after tri-modality therapy for carcinomas of the superior pulmonary sulcus. Virchows Arch 2013;462:547-56. [Crossref] [PubMed]
- Thunnissen FB, den Bakker MA. Implications of frozen section analyses from bronchial resection margins in NSCLC. Histopathology 2005;47:638-40. [Crossref] [PubMed]
- Bahce I, Vos CG, Dickhoff C, et al. Metabolic activity measured by FDG PET predicts pathological response in locally advanced superior sulcus NSCLC. Lung Cancer 2014;85:205-12. [Crossref] [PubMed]
- Ahn H, Lee KH, Kim J, et al. Diameter of the Solid Component in Subsolid Nodules on Low-Dose Unenhanced Chest Computed Tomography: Measurement Accuracy for the Prediction of Invasive Component in Lung Adenocarcinoma. Korean J Radiol 2018;19:508-15. [Crossref] [PubMed]
- Aherne EA, Plodkowski AJ, Montecalvo J, et al. What CT characteristics of lepidic predominant pattern lung adenocarcinomas correlate with invasiveness on pathology? Lung Cancer 2018;118:83-9. [Crossref] [PubMed]
- Lampen-Sachar K, Zhao B, Zheng J, et al. Correlation between tumor measurement on Computed Tomography and resected specimen size in lung adenocarcinomas. Lung Cancer 2012;75:332-5. [Crossref] [PubMed]
- Ridge CA, Huang J, Cardoza S, et al. Comparison of Multiplanar Reformatted CT Lung Tumor Measurements to Axial Tumor Measurement Alone: Impact on Maximal Tumor Dimension and T Stage. AJR Am J Roentgenol 2013;201:959-63. [Crossref] [PubMed]
- Thunnissen E, Blaauwgeers HJ, de Cuba EM, et al. Ex Vivo Artifacts and Histopathologic Pitfalls in the Lung. Arch Pathol Lab Med 2016;140:212-20. [Crossref] [PubMed]
- Thunnissen E, Beliën JA, Kerr KM, et al. In compressed lung tissue microscopic sections of adenocarcinoma in situ may mimic papillary adenocarcinoma. Arch Pathol Lab Med 2013;137:1792-7. [Crossref] [PubMed]
- Werner M, Chott A, Fabiano A, et al. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol 2000;24:1016-9. [Crossref] [PubMed]
- Cross SS, Start RD, Smith JH. Does delay in fixation affect the number of mitotic figures in processed tissue? J Clin Pathol 1990;43:597-9. [Crossref] [PubMed]
- Khoury T, Sait S, Hwang H, et al. Delay to formalin fixation effect on breast biomarkers. Mod Pathol 2009;22:1457-67. [Crossref] [PubMed]
- Bussolati G, Annaratone L, Medico E, et al. Formalin fixation at low temperature better preserves nucleic acid integrity. PLoS One 2011;6:e21043. [Crossref] [PubMed]


