
The obesity paradox in lung cancer: is there a missing biological link?
Obesity is a well-established risk factor for cardiovascular diseases (1) and type II diabetes (2). It has also been long associated with increased risk and mortality of most cancer types (3). Paradoxically, high body mass index (BMI) has been linked with both a reduced risk of lung cancer and better overall outcome of lung cancer patients. The mechanism for the paradoxical benefit of obesity observed consistently in multiple cohort studies, termed as the “obesity paradox”, remains unclear. The hypotheses of a confounding effect by smoking or reverse causation due to cancer-associated weight loss have been already considered as possible reasonable interpretations, but have failed to explain the lung cancer obesity paradox in its entirety (4,5).
Thus, the question still lingers: Is there a biological mechanism that could explain, at least partially, the obesity paradox in lung cancer?
Previous studies have shown that the development and progression of human malignancies is favored by deregulated cell metabolism which is now considered as one of the hallmarks of cancer (6). Furthermore, several cancer-related genes are shown to have an important role in metabolic control (6,7). In lung cancer in particularly, the fat mass and obesity-associated (FTO) gene which is linked with increased BMI has also been associated with a decreased risk of this malignancy (8).
Interestingly, the recent research by Nagano et al. (9) shows that DPYSL4, a direct target of p53, regulates energy metabolism both in adipocytes and cancer cells. The investigators performed RNA-seq and ChIP sequencing in lung cancer cells and preadipocytes, to identify a set of p53-inducable genes which are implicated in energy metabolism in both biological backgrounds. A total of 60 genes appeared to be common p53-mediated downstream effectors in both cell types, a fact that broadens the already known functions of p53. Among the tested genes, DPYSL4 was significantly induced in lung cancer cells expressing p53 and preadipocytes, a fact that militates in favor of DPYSL4 being important for p53 response in tumor cells and altered energy metabolism. DPYSL4 gene, which belongs to the collapsin response mediator protein (CRMP) family, was previously identified as a p53 direct target associated with apoptosis induction in response to DNA damage (10). The authors have also assessed the subcellular localization of DPYSL4 protein, in an attempt to unravel its function. DPYSL4 was found to have both cytosolic and mitochondrial localization, particularly in association with specific mitochondrial respiratory supercomplex assemblies. Based on the notion that cancer cells rely primarily on glycolysis to produce energy, rather on oxidative phosphorylation (OXPHOS), the investigators showed that DPYSL4 directly affects OXPHOS and ATP production in mitochondria. In addition, DPYSL4 silencing resulted in the elevation of the Krebs cycle intermediates, succinate, fumarate, and malate, indicating that DPYSL4 may directly influence the regulation of the TCA cycle rather than upstream steps of the glycolytic pathway. Several lines of biochemical and genetic evidence support roles for fumarate, succinate, in cellular transformation and oncogenesis. Furthermore, DPYSL4 overexpression in cancer cells and preadipocytes up-regulated ATP production and oxygen consumption (favoring OXPHOS), while DPYSL4 knockdown using siRNA or CRISPR/Cas9 down-regulated energy production (9).
The authors have also investigated the physiological effects of DPYSL4 on cancer cell proliferation both in vitro and in vivo. Overexpression of p53 or DPYSL4 in lung cancer cell lines reduced their invasive ability on Matrigel matrices in vitro. Additionally, in vivo studies, using mouse xenograft and lung metastasis models showed that DPYSL4 expression significantly reduces tumor growth and lung metastasis. One of the most interesting findings of the study by Nagano et al. is the fact that p53 was significantly up-regulated in adipocytes of obese patients, and the mRNA levels of DPYSL4, CDKN1A, MCP1, and IFNg were also higher in adipose tissues of obese compared to non-obese patients. In addition, CD68-positive cells exhibited marked infiltration in adipose tissue of obese patients and DPYSL4 is positively correlated with BMI and INF-γ levels (9). When taken together, these results indicate the existence of a link between p53-inducible DPYSL4 and the pathophysiology of cancer and metabolic disorders, possibly via its energy-regulating function.
The very interesting findings of the aforementioned study, showing that p53 and its direct targets are involved in the pathogenesis of both cancer and obesity, prompted us to question if and how adipose tissue can affect the metabolic gene expression in lung cancer.
The conventional role of adipose tissue as a strictly energy storage depot has been revisited recently. Adipose tissue is now considered as a fully active endocrine organ that secretes hormones (adipokines) influencing several pathophysiological processes, including cancer (11,12). The most well studied adipokines are leptin, adiponectin, chemerin (11) and omentin (13,14). The cross talk between adipose tissue and cancer cells has been previously described (11,15). For lung cancer, a communication between adipose tissue and cancer cells (Figure 1) that would explain the obesity paradox, should be mediated through an adipokine increased in obese individuals that at the same time exerts anti-tumor properties. Leptin is increased in obese individuals, but has been shown to have tumor promoting properties. On the other hand, adiponectin has tumor-suppressive properties and lung cancer cells have receptors that bind this adipokine, but its levels decrease with increased BMI (11). Omentin seems to follow the same pattern as adiponectin (13,14). Data about chemerin are controversial. Chemerin serum levels have been positively correlated with increased BMI (16) and its expression has been demonstrated to have tumor-suppressive properties in lung cancer and to be associated with favorable prognosis in a mechanism that involves the increased infiltration of natural killer cells that attack the tumor (17). On the other hand, increased chemerin serum levels are associated with poor prognosis in lung cancer (18). It is difficult to imagine how one of the abovementioned adipokines could result in an increase of DPYSL4 or any other tumor suppressor gene in the lung cancer tissue, although some of them, like adiponectin and omentin, have been previously shown to induce p53 signaling (14,19) and thus could potentially result to DPYSL4 overexpression.
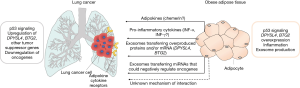
Obesity-related inflammation could be a possible explanation to the obesity paradox in lung cancer. Obese adipose tissue is a source of circulating pro-inflammatory cytokines interleukin (IL)-6, IL-8, IL-1β, tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), chemokine (C-C motif) ligand 2 (CCL2), and CCL5 and interferon (IFN)-γ (20). Nagano et al. showed (9) that there is a pathophysiological relationship between p53 activation and inflammatory cytokines, leading to the up-regulation of DPYSL4 in human adipose tissue of obese patients. It would be intriguing to hypothesize that a circulating pro-inflammatory cytokine could induce a p53-related DPYSL4 overexpression to the lung cancer cells of obese individuals that will ultimately lead to tumor suppression.
Another route of transport of p53 induced molecules with antitumor properties from adipocytes to the target tissue (lung cancer cells) would be through extracellular vesicles (EVs). Secreted EVs could horizontally transfer metabolic changes between adipocytes and remote recipient cells. EVs can alter the molecular properties of recipient cells though the acquisition of new proteins (receptors, enzymes) or even genetic material (mRNAs, miRNAs regulating gene expression) from the adipocyte cells of origin, thereby serving as a novel class of adipokines. In fact, this horizontal transfer is reinforced in obesity due to the larger number of exosomes secreted and the stronger effect of each individual exosome. It should be noted that both the inflammation- and EV-related theories have been previously used to explain positive associations between BMI and the initiation/progression of other cancers (20).
In kidney cancer, where the obesity paradox is also present (21), a metabolic gene regulation pattern that provides a somewhat clearer mechanistic insight into this favorable effect of obesity in disease prognosis has been proposed. Hakimi et al. (22) demonstrated that kidney cancer tissue from obese patients had a different metabolic gene expression pattern. More precisely, the fatty acid synthase (FASN) gene was downregulated in obese, compared to normal weight, kidney cancer patients and that this downregulation offers a survival benefit for obese patients. The authors assumed that TRAIL (TNF-related apoptosis-inducing ligand) could be a possible link that explains how obesity affects the metabolic gene expression of kidney tumors. TRAIL is increased in the serum of obese individuals and can lead to downregulation of FASN gene expression (22).
Taking into consideration the data presented above, the expression profile of metabolic-related genes that also have tumor suppressor properties (DPYSL4, BTG2), identified in the work of Nagano et al. (9), could be investigated in obese and non-obese lung cancer patients in conjunction with disease prognosis. In this future study it would be essential to include measurements of major adipokines, inflammatory cytokines, exosomes, as well as the p53 mutation status of tumors. Histology should also be taken into account given that obesity tends to affect each lung cancer subtype differently (4).
The findings of Nagano et al. encourage studies that would further evaluate the therapeutic potential of altering the metabolic profile and reactivating p53 in lung cancer. The latter is a highly relevant therapeutic strategy for lung cancer where mutations have been reported in up to 80% of samples. Several molecules have been reported to be able to restore the wild-type function and thus reverse the oncogenic properties of mutant p53. Among these, the most widely investigated is the low molecular weight molecule, named PRIMA-1 {2,2-bis(hydroxymethyl)-1-azabicyclo[2,2,2] octan-3-one}. Both in vitro and in vivo studies in lung cancer have shown that PRIMA-1 synergizes with cisplatin to achieve tumor regression (23). Furthermore, disrupting the “oncogenic” metabolism of lung cancer cells represents a very attractive therapeutic approach. This could be accomplished by shifting the balance of the hyperactive aerobic glycolysis independent of oxygen availability (Warburg effect) towards OXPHOS. Preliminary findings show that targeting key enzymes of aerobic glycolysis with small molecule inhibitors interrupts the proliferation and survival of lung cancer cells (24). A more applicable way to target the Warburg effect would be via a ketogenic diet (KD), which is a high-fat/low-glucose/normal-protein diet. Apart from by limiting the availability of glucose to cancer cells and thus directly disrupting the Warburg effect, KDs seem to exert their adjuvant cancer therapeutic effect by taking into advantage the fact that many cancers cannot metabolize ketone bodies due to mitochondrial dysfunctions. Two preclinical studies have already provided evidence for an anti-tumor effect of KDs in lung cancer (25).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898-918. [Crossref] [PubMed]
- Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014;370:233-44. [Crossref] [PubMed]
- Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol 2015;16:36-46. [Crossref] [PubMed]
- Yu D, Zheng W, Johansson M, et al. Overall and Central Obesity and Risk of Lung Cancer: A Pooled Analysis. J Natl Cancer Inst 2018;110:831-42. [Crossref] [PubMed]
- Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2017;104:52-7. [Crossref] [PubMed]
- Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2012;2:881-98. [Crossref] [PubMed]
- Furuta E, Okuda H, Kobayashi A, et al. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta 2010;1805:141-52. [PubMed]
- Brennan P, McKay J, Moore L, et al. Obesity and cancer: Mendelian randomization approach utilizing the FTO genotype. Int J Epidemiol 2009;38:971-5. [Crossref] [PubMed]
- Nagano H, Hashimoto N, Nakayama A, et al. p53-inducible DPYSL4 associates with mitochondrial supercomplexes and regulates energy metabolism in adipocytes and cancer cells. Proc Natl Acad Sci U S A 2018;115:8370-5. [Crossref] [PubMed]
- Kimura J, Kudoh T, Miki Y, et al. Identification of dihydropyrimidinase-related protein 4 as a novel target of the p53 tumor suppressor in the apoptotic response to DNA damage. Int J Cancer 2011;128:1524-31. [Crossref] [PubMed]
- Ntikoudi E, Kiagia M, Boura P, et al. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev 2014;40:22-30. [Crossref] [PubMed]
- Zwezdaryk K, Sullivan D, Saifudeen Z. The p53/Adipose-Tissue/Cancer Nexus. Front Endocrinol (Lausanne) 2018;9:457. [Crossref] [PubMed]
- de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007;56:1655-61. [Crossref] [PubMed]
- Zhang YY, Zhou LM. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol 2013;698:137-44. [Crossref] [PubMed]
- Zhang X, Liu Y, Shao H, et al. Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. J Thorac Oncol 2017;12:1478-88. [Crossref] [PubMed]
- Sledzinski T, Korczynska J, Hallmann A, et al. The increase of serum chemerin concentration is mainly associated with the increase of body mass index in obese, non-diabetic subjects. J Endocrinol Invest 2013;36:428-34. [PubMed]
- Zhao S, Li C, Ye Y, et al. Expression of Chemerin Correlates With a Favorable Prognosis in Patients With Non-Small Cell Lung Cancer. Laboratory Medicine 2011;42:553-7. [Crossref]
- Xu CH, Yang Y, Wang YC, et al. Prognostic significance of serum chemerin levels in patients with non-small cell lung cancer. Oncotarget 2017;8:22483-9. [PubMed]
- Mistry T, Digby JE, Desai KM, et al. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int 2008;101:1317-22. [Crossref] [PubMed]
- Robado de Lope L, Alcibar OL, Amor Lopez A, et al. Tumour-adipose tissue crosstalk: fuelling tumour metastasis by extracellular vesicles. Philos Trans R Soc Lond B Biol Sci 2018.373. [PubMed]
- Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer 2013;132:625-34. [Crossref] [PubMed]
- Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 2013;105:1862-70. [Crossref] [PubMed]
- Duffy MJ, Synnott NC, McGowan PM, et al. p53 as a target for the treatment of cancer. Cancer Treat Rev 2014;40:1153-60. [Crossref] [PubMed]
- Li XB, Gu JD, Zhou QH. Review of aerobic glycolysis and its key enzymes - new targets for lung cancer therapy. Thorac Cancer 2015;6:17-24. [Crossref] [PubMed]
- Weber DD, Aminazdeh-Gohari S, Kofler B. Ketogenic diet in cancer therapy. Aging (Albany NY) 2018;10:164-5. [Crossref] [PubMed]


