Transcatheter, sutureless and conventional aortic-valve replacement: a network meta-analysis of 16,432 patients
Introduction
Aortic stenosis (AS) is a valvular cardiac disease with an increasing incidence in an aging population (1). Conventional aortic valve replacement (CAVR) has historically been the gold standard for surgical intervention of AS, however approximately a third of patients with AS present with a high degree of co-morbidities (2) rendering them unsuitable for CAVR.
Technological advances have focused on the development of minimally invasive techniques to expand interventions to patients with AS who are deemed inoperable. These new treatment alternatives include sutureless aortic valve replacement (SL-AVR) and transcatheter aortic valve implantation (TAVI) (3-6). Recent randomised controlled trials (RCTs) have found outcomes of TAVI (7) and SL-AVR to be non-inferior to CAVR amongst high-risk patients (8). Multi-arm analyses comparing perioperative outcomes amongst TAVI, SL-AVR and CAVR, which could potentially lend support to particular recommendations, are currently lacking.
We present a Bayesian network analysis comparing Valve Academic Research Consortium-2 clinical outcomes between TAVI, SL-AVR and CAVR. Findings from this study are of particular importance given the drive towards use of TAVI and SL-AVR in place of CAVR amongst all-comers in the treatment of AS.
Methods
Literature search strategy
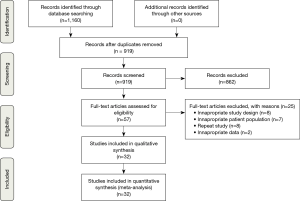
Five electronic databases were searched including PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club and the Database of Abstracts of Review of Effectiveness (DARE). To minimise the risk of overlooking relevant studies and given the wide variety of procedural nomenclature, it was necessary to combine a large number of key words and MeSH terms. This constituted the terms “sutureless” or “transcatheter” or “transfemoral” or “transapical” or “trans-subclavian” or “conventional” or “standard” or “minimally invasive” and “aortic valve” or “aortic-valve” and “implantation” or “replacement” or “procedure” or “treatment” and “aortic stenosis” and “randomised” or “propensity” or “trial”. Reference lists of relevant literature were examined for any further studies. Figure 1 depicts a PRISMA flow diagram highlighting the overall search strategy.
Outcome measures
Baseline characteristics for both pre- and post-propensity score matching cohorts were recorded, as well as all postoperative outcomes within the given timeframes. For each postoperative outcome published, criteria from the Valve Academic Research Consortium-2 (VARC-2) (9) were applied to maintain consistency and validity of results. In line with VARC-2 recommendations, mortality was only recorded if it was 30-day postoperative all-cause mortality. The VARC-2 guideline does not place similar 30-day time periods on the reporting of other postoperative complications, as such, this data was retained.
Eligibility criteria
Only propensity score matching studies and RCTs published in English were deemed eligible to be included in the analysis. Studies were included if they recorded specific postoperative outcomes following an AVR. Selection criteria was non-discriminant towards studies focusing on patient populations deemed low, medium or high surgical risk; however, a publication was excluded if the authors focused on AVR outcomes in the presence of one or more specific co-morbidities. If there were multiple studies published on the same patient population, only the most recent literature was included. All case reports, expert opinions, singe-arm studies and presentations were excluded. All studies on non-human subjects were removed.
Data extraction
Two reviewers independently appraised studies using a standard form and extracted data on methodology and outcome measures. Additionally, quality of studies was appraised using assessment criteria recommended by the Centre for Evidence Based Medicine (University of Oxford) (Table S1). For quantitative baseline characteristics, only data given as a mean and standard deviation or as a median and range were recorded. Discrepancies between reviewers were resolved by discussion and consensus.
Full table
Statistical analysis
Baseline patient characteristics were assessed through pairwise analysis of odds ratios for dichotomous data and the difference of means for continuous data.
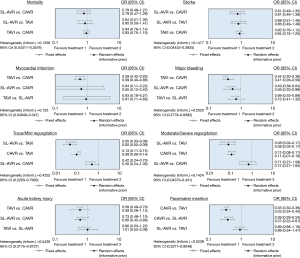
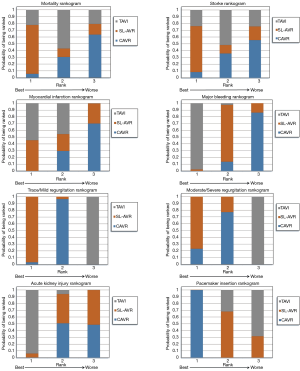
Clinical postoperative outcomes were examined using odds ratios, specifically random effects with informative priors to best minimise the impact of the diversity of the assorted patient populations and designs for each study. Bayesian analysis was implemented due to its ability to simultaneously compare multiple treatment options and for its greater flexibility. The analyses were executed using the Bayesian Markov Chain Monte Carlo method in WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, UK) through the conduit of the Microsoft Excel based macro NetMetaXL 1.6.1 (Canadian Agency for Drugs and Technologies in Health) (10). A convergence test for each analysis was conducted by checking whether the Monte Carlo error was less than 5% of the standard deviation of the effect estimates or the variance between the studies. Convergence was achieved for all analyses at 20,000 “burn in” runs and 30,000 model runs. Furthermore, NetMetaXL allows for rank probabilities to be plotted against the possible ranks for a treatment to result in the production of a graphical “rankogram” (11). This method of visually representing probabilities was combined with a surface under the cumulative ranking line for each surgical intervention (SUCRA). For example, a SUCRA of 0.5 means that there is a 50% chance that the respective intervention is the best option in achieving the lowest rate of an undesirable clinical outcome. The forest plots and rankograms generated from the analysis are presented in Figures 2 and 3 respectively.
Results
Of the 32 studies that met inclusion criteria, seven were RCTs and 25 propensity matched studies; recording outcomes for 16,432 patients (Table 1).
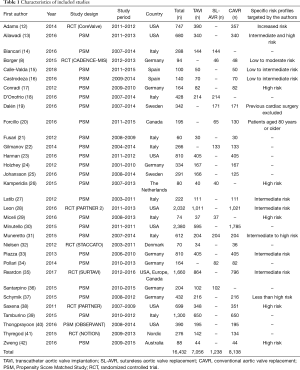
Full table
Of these patients, 8,138 patients underwent CAVR, 7,056 received TAVI and 1,238 received SL-AVR. Various routes (transfemoral, subclavian, transapical) of transcatheter access were reported in the literature, however this data was not comprehensively published. Of note, two studies focused entirely on transapical transcatheter approaches (n=201) (24,32).
Eight postoperative outcomes were identified from the VARC-2 consensus document to be suitable for the network analysis due to their consistent reporting across the range of studies. These included 30-day all-cause mortality, major bleeding or bleeding requiring surgical re-exploration, postoperative cerebrovascular accident (CVA), transient ischaemic attack (TIA), acute kidney injury (AKI), renal failure, rates of mild/trace paravalvular leakage, rates of moderate/severe paravalvular leakage, myocardial infarction and permanent pacemaker (PPM) implantation.
Baseline characteristics
Patients who received SL-AVR in studies compared against CAVR had significantly higher rates of diabetes mellitus (OR 0.64, 95% CI: 0.44–0.93, P=0.02). No other differences were found in the baseline characteristics for any matched patient population (Table 2).
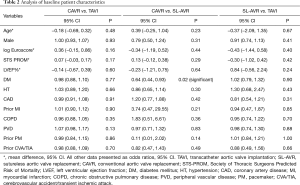
Full table
Mortality
The network meta-analysis yielded no significant differences. The modelling suggested that SL-AVR had the highest probability of producing the lowest rate of 30-day mortality of the three interventions with a SUCRA of 78.20%. Heterogeneity was low (τ2=0.1356).
CVA/TIA
The network meta-analysis yielded no significant differences. SUCRA was highest for SL-AVR at 73.83% and heterogeneity was low (τ2=0.1477).
Myocardial infarction
TAVI was significantly associated with a 41% reduction in incidence of postoperative myocardial infarction when compared to CAVR (OR 0.59, 95% CI: 0.40–0.86). There were no other significant associations. SUCRA was highest for TAVI at 77.13%. Heterogeneity was low (τ2=0.125).
Major bleeding
TAVI was significantly associated with a 59% reduced incidence of postoperative major bleeding compared to CAVR (OR 0.41, 95% CI: 0.28–0.59). Similarly, SL-AVR was significantly associated with a 44% reduction in major bleeding compared to CAVR (OR 0.56, 95% CI: 0.30–0.99). The modelling indicated that TAVI had the highest probability of producing the lowest rates of bleeding. SUCRA for TAVI was 98.83% and heterogeneity was high (τ2=0.5525).
Trace/mild paravalvular regurgitation
SL-AVR and CAVR were significantly associated with a reduction in the occurrence of trace or mild levels of regurgitation when compared to TAVI, 95% (OR 0.05, 95% CI: 0.02–0.09) and 91% (OR 0.09, 95% CI: 0.06–0.14) respectively. The highest SUCRA was for SL-AVR at 98.37% and heterogeneity was high (τ2=0.4252).
Moderate/severe paravalvular regurgitation
SL-AVR and CAVR were significantly associated with a reduction in the occurrence of moderate to severe postoperative aortic paravalvular regurgitation when compared to TAVI, 92% (OR 0.08, 95% CI: 0.03–0.17) and 89% (OR 0.11, 95% CI: 0.07–0.16) respectively. The highest SUCRA was for SL-AVR at 88.53% and heterogeneity was low (τ2=0.1424).
Acute kidney injury
SL-AVR was associated with a 40% reduction in postoperative AKI rates compared to CAVR (OR 0.60, 95% CI: 0.42–0.86). No other significant associations were reported. However, the modelling indicated that TAVI had the highest SUCRA of 96.89%. Heterogeneity was high (τ2=0.5426).
Pacemaker implantation
CAVR was significantly associated with a 67% reduction in postoperative PPM when compared to TAVI (OR 0.33, 95% CI: 0.24–0.45) and a 63% reduction when compared to SL-AVR (OR 0.37; 95% CI: 0.22–0.61). The highest SUCRA was for CAVR at 99.99% and heterogeneity was high (τ2=0.5238).
Discussion
In the present study, we sought to compare postoperative outcomes of TAVI, SL-AVR and CAVR by means of a network meta-analysis using Bayesian Markov chain Monte Carlo modelling. Our analysis has several significant findings. TAVI was associated with reduced rates of postoperative myocardial infarction and major bleeding when compared to CAVR but had higher rates for all grades of paravalvular regurgitation when compared to SL-AVR and CAVR. SL-AVR was associated with reduced rates of postoperative major bleeding and AKI when compared to CAVR. However, compared to CAVR, both TAVI and SL-AVR had significantly higher rates of conduction disturbance requiring PPM. There were no differences with regards to 30-day all-cause mortality or postoperative stroke between the groups. The pairwise analysis found little difference between the preoperative patient populations.
Compared to other meta-analyses, our network analysis differs in rates of clinically relevant perioperative complications. Whereas Khan et al. (43) found no difference in bleeding when TAVI was compared with CAVR, our findings of reduced rates of major bleeding with TAVI are consistent with findings from Tam et al. (44). These differences may be attributed to variance in sample size and study type, when comparing the meta-analysis of observational studies by Khan et al. with a sample size of 420, to the meta-analysis of RCTs by Tam et al., total sample size 8,234, and our study of 16,432 patients.
Our study demonstrates that compared to CAVR, the TAVI cohort experienced a 41% reduction in postoperative myocardial infarction. However, the VARC-2 criteria for diagnosis of myocardial infarction may overestimate this outcome due to direct procedural related myocardial injury confounding findings of increased cardiac troponins and creatine kinase MB (45). Furthermore, compared to CAVR, SL-AVR was significantly associated with reduced rates of postoperative AKI grade 2 or 3, however AKI had the highest level of heterogeneity, likely secondary to discrepancies in grading between studies.
We observed significantly higher rates of all grades of paravalvular regurgitation for TAVI when compared to SL-AVR or CAVR. Three studies (24,26,27) included rates for aortic regurgitation but were excluded from analysis as they did not specify type of leakage (transvalvular, paravalvular or both). Conflicting data exists in regards to the long-term outcomes for postoperative aortic regurgitation (46). Results of the PARTNER trial at 2 years suggest that even a mild degree of aortic regurgitation significantly decreases patient survival (47). Aortic regurgitation following TAVI implantation may be secondary to non-uniform calcified native valve compression against the aortic wall following TAVI deployment or suboptimal balloon inflation. In contrast, the ability to remove valve leaflets, decalcify the annulus and size and implant under direct vision have been attributed as reasons for reduced postoperative aortic regurgitation for SL-AVR and CAVR (48). Heterogeneity exists in the grading schemes of paravalvular regurgitation (49) and it is unclear whether VARC-2 definitions were universally used. Technological developments in TAVI technology include the introduction of a polyethylene terephthalate outer skirting in the SAPIEN 3 prosthesis (Edwards Lifesciences, Irvine, California) with the goal of increasing contact and adhesion of the valve against the aortic annulus (50). Clinical results suggest that the SAPIEN 3 prosthesis is associated with a lower incidence of paravalvular leak compared to SAPIEN XT (Edwards Lifesciences) and CoreValve EvolutR (Medtronic, Minneapolis, Minnesota) (51). Future larger studies with longer follow-up will be required to demonstrate whether these differences have a significant clinical effect.
Significant heterogeneity for PPM exists between studies with 34.1% of TAVI patients in the NOTION trial receiving a PPM (41) in contrast to 3.8% in the PARTNER trial (52). Variance in studies may be attributed to the different devices implanted and implantation technique (53). Other meta-analyses of newer generation TAVI valves appear to still indicate relatively high rates of PPM of approximately 16.2% (54). In the present study, there was a 67% reduction in the rate of PPM with CAVR compared to SL-AVR. It is suggested that excessive removal of calcified cusps during the implantation of a SL-AVR is predictive of PPM (55). Overall, the higher risk of PPM presents an important consideration in balancing the risks and benefits of minimally invasive approaches.
The results of the original PARTNER trial and the CoreValve US Pivotal Trial have led to the recognition of TAVI as the procedure of the choice for inoperable patients and an alternative to CAVR in high risk patients (52). However, when considering intermediate risk patients, Muneretto et al. (56) demonstrate in a retrospective multicohort study that TAVI significantly increased early and late morbidity and mortality when compared with SL-AVR and CAVR; moreover, use of TAVI was identified as an independent predictor for all-cause mortality in intermediate risk patients.
Currently, multiple trials are underway including the PARTNER 3 (Placement of Aortic Transcatheter Trial 3, NCT02675114) to investigate SAPIEN 3 TAVI as compared to CAVR for low risk patients as well as the PERSIST-AVR trial (Perceval Sutureless Implant Versus Standard-Aortic Valve Replacement, NCT02673697). It is with anticipation that we await the long-term outcomes from these multicentre, randomised trials which we hope will provide new insight into the role of TAVI and SL-AVR, if any, in intermediate and low risk patients.
Limitations
Pooling of data from trials with different inclusion criteria, eras, design, patient surgical risks, concomitant procedures, follow-up duration with variable attrition rates and variable definition and validation of endpoints contributes to the heterogeneity observed between the studies. Additionally, we refined the multitude of techniques for AVR into the three broad categories of TAVI, SL-AVR and CAVR which were not able to account for variable practices between centres, differences in vascular access and types of valves implanted. We acknowledge that this heterogeneity in study population and different indications for each type of valve is a fundamental limitation that cannot be addressed due to inability to extract sufficient detail from the pooled data.
Conclusions
The inclusion of RCTs and propensity-matched studies are strengths in the present meta-analysis. However, there are several key limitations that merit consideration. This network meta-analysis demonstrates no differences in perioperative mortality or stroke between patients who received TAVI, SL-AVR or CAVR interventions for their AS. Minimally invasive surgical and percutaneous interventions for severe AS are a viable alternative to CAVR in selected patients. However, TAVI is associated with increased paravalvular regurgitation, whereas TAVI and SL-AVR are associated with increased conduction disturbances compared to CAVR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Supino PG, Borer JS, Preibisz J, et al. The epidemiology of valvular heart disease: a growing public health problem. Heart Fail Clin 2006;2:379-93. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J 2011;32:198-204. [Crossref] [PubMed]
- Breitenbach I, Wimmer-Greinecker G, Bockeria LA, et al. Sutureless aortic valve replacement with the Trilogy Aortic Valve System: Multicenter experience.
- Sadowski J, Kapelak B, Pfitzner R, et al. Sutureless aortic valve bioprosthesis '3F/ATS Enable'--4.5 years of a single-centre experience. Kardiologia polska 2009;67:956-63. [PubMed]
- Englberger L, Carrel TP, Doss M, et al. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg 2014;148:1681-7. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25. [Crossref] [PubMed]
- Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. [Crossref] [PubMed]
- Brown S, Hutton B, Clifford T, et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses--an overview and application of NetMetaXL. Syst Rev 2014;3:110. [Crossref] [PubMed]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Ailawadi G, LaPar DJ, Speir AM, et al. Contemporary Costs Associated With Transcatheter Aortic Valve Replacement: A Propensity-Matched Cost Analysis. Ann Thorac Surg 2016;101:154-60; discussion 160. [Crossref] [PubMed]
- Biancari F, Barbanti M, Santarpino G, et al. Immediate outcome after sutureless versus transcatheter aortic valve replacement. Heart Vessels 2016;31:427-33. [Crossref] [PubMed]
- Calle-Valda CM, Aguilar R, Benedicto A, et al. Outcomes of Aortic Valve Replacement According to Surgical Approach in Intermediate and Low Risk Patients: A Propensity Score Analysis. Heart Lung Circ 2018;27:885-92. [Crossref] [PubMed]
- Castrodeza J, Amat-Santos IJ, Blanco M, et al. Propensity score matched comparison of transcatheter aortic valve implantation versus conventional surgery in intermediate and low risk aortic stenosis patients: A hint of real-world. Cardiol J 2016;23:541-51. [PubMed]
- Conradi L, Seiffert M, Treede H, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a propensity score analysis in patients at high surgical risk. J Thorac Cardiovasc Surg 2012;143:64-71. [Crossref] [PubMed]
- D'Onofrio A, Salizzoni S, Rubino AS, et al. The rise of new technologies for aortic valve stenosis: A comparison of sutureless and transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2016;152:99-109.e2. [Crossref] [PubMed]
- Dalén M, Biancari F, Rubino AS, et al. Aortic valve replacement through full sternotomy with a stented bioprosthesis versus minimally invasive sternotomy with a sutureless bioprosthesis. Eur J Cardiothorac Surg 2016;49:220-7. [Crossref] [PubMed]
- Forcillo J, Bouchard D, Nguyen A, et al. Perioperative outcomes with sutureless versus stented biological aortic valves in elderly persons. J Thorac Cardiovasc Surg 2016;151:1629-36. [Crossref] [PubMed]
- Fusari M, Bona V, Muratori M, et al. Transcatheter vs. surgical aortic valve replacement: a retrospective analysis assessing clinical effectiveness and safety. J Cardiovasc Med (Hagerstown) 2012;13:229-41. [Crossref] [PubMed]
- Gilmanov D, Miceli A, Ferrarini M, et al. Aortic valve replacement through right anterior minithoracotomy: can sutureless technology improve clinical outcomes? Ann Thorac Surg 2014;98:1585-92. [Crossref] [PubMed]
- Hannan EL, Samadashvili Z, Stamato NJ, et al. Utilization and 1-Year Mortality for Transcatheter Aortic Valve Replacement and Surgical Aortic Valve Replacement in New York Patients With Aortic Stenosis: 2011 to 2012. JACC Cardiovasc Interv 2016;9:578-85. [Crossref] [PubMed]
- Holzhey DM, Shi W, Rastan A, et al. Transapical versus conventional aortic valve replacement--a propensity-matched comparison. Heart Surg Forum 2012;15:E4-8. [Crossref] [PubMed]
- Johansson M, Nozohoor S, Bjursten H, et al. Late survival and heart failure after transcatheter aortic valve implantation. Asian Cardiovasc Thorac Ann 2016;24:318-25. [Crossref] [PubMed]
- Kamperidis V, van Rosendael PJ, de Weger A, et al. Surgical sutureless and transcatheter aortic valves: hemodynamic performance and clinical outcomes in propensity score-matched high-risk populations with severe aortic stenosis. JACC Cardiovasc Interv 2015;8:670-7. [Crossref] [PubMed]
- Latib A, Maisano F, Bertoldi L, et al. Transcatheter vs surgical aortic valve replacement in intermediate-surgical-risk patients with aortic stenosis: a propensity score-matched case-control study. Am Heart J 2012;164:910-7. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Miceli A, Gilmanov D, Murzi M, et al. Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients. Eur J Cardiothorac Surg 2016;49:960-5. [Crossref] [PubMed]
- Minutello RM, Wong SC, Swaminathan RV, et al. Costs and in-hospital outcomes of transcatheter aortic valve implantation versus surgical aortic valve replacement in commercial cases using a propensity score matched model. Am J Cardiol 2015;115:1443-7. [Crossref] [PubMed]
- Muneretto C, Alfieri O, Cesana BM, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in "real-world" patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg 2015;150:1570-7; discussion 1577-9. [Crossref] [PubMed]
- Nielsen HH, Klaaborg KE, Nissen H, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012;8:383-9. [Crossref] [PubMed]
- Piazza N, Kalesan B, van Mieghem N, et al. A 3-center comparison of 1-year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate-risk surgical patients. JACC Cardiovasc Interv 2013;6:443-51. [Crossref] [PubMed]
- Pollari F, Santarpino G, Dell'Aquila AM, et al. Better short-term outcome by using sutureless valves: a propensity-matched score analysis. Ann Thorac Surg 2014;98:611-6; discussion 616-7. [Crossref] [PubMed]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2017;376:1321-31. [Crossref] [PubMed]
- Santarpino G, Pfeiffer S, Jessl J, et al. Clinical Outcome and Cost Analysis of Sutureless Versus Transcatheter Aortic Valve Implantation With Propensity Score Matching Analysis. Am J Cardiol 2015;116:1737-43. [Crossref] [PubMed]
- Schymik G, Heimeshoff M, Bramlage P, et al. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc Interv 2015;86:738-44. [Crossref] [PubMed]
- Saxena A, Dinh DT, Yap CH, et al. Critical analysis of early and late outcomes after isolated coronary artery bypass surgery in elderly patients. Ann Thorac Surg 2011;92:1703-11. [Crossref] [PubMed]
- Tamburino C, Barbanti M, D'Errigo P, et al. 1-Year Outcomes After Transfemoral Transcatheter or Surgical Aortic Valve Replacement: Results From the Italian OBSERVANT Study. J Am Coll Cardiol 2015;66:804-12. [Crossref] [PubMed]
- Thongprayoon C, Cheungpasitporn W, Srivali N, et al. AKI after Transcatheter or Surgical Aortic Valve Replacement. J Am Soc Nephrol 2016;27:1854-60. [Crossref] [PubMed]
- Thyregod HG, Steinbruchel DA, Ihlemann N, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184-94. [Crossref] [PubMed]
- Zweng I, Shi WY, Palmer S, et al. Transcatheter versus Surgical Aortic Valve Replacement in High-risk Patients: A propensity-score matched analysis. Heart Lung Circ 2016;25:661-7. [Crossref] [PubMed]
- Khan AR, Khan S, Riaz H, et al. Efficacy and safety of transcatheter aortic valve replacement in intermediate surgical risk patients: A systematic review and meta-analysis. Catheter Cardiovasc Interv 2016;88:934-44. [Crossref] [PubMed]
- Tam DY, Vo TX, Wijeysundera HC, et al. Transcatheter vs Surgical Aortic Valve Replacement for Aortic Stenosis in Low-Intermediate Risk Patients: A Meta-analysis. Can J Cardiol 2017;33:1171-9. [Crossref] [PubMed]
- Rodés-Cabau J, Gutierrez M, Bagur R, et al. Incidence, predictive factors, and prognostic value of myocardial injury following uncomplicated transcatheter aortic valve implantation. J Am Coll Cardiol 2011;57:1988-99. [Crossref] [PubMed]
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585-95. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Shrestha M, Folliguet T, Meuris B, et al. Sutureless Perceval S aortic valve replacement: a multicenter, prospective pilot trial. J Heart Valve Dis 2009;18:698-702. [PubMed]
- Hahn RT, Pibarot P, Weissman NJ, et al. Assessment of paravalvular aortic regurgitation after transcatheter aortic valve replacement: intra-core laboratory variability. J Am Soc Echocardiogr 2015;28:415-22. [Crossref] [PubMed]
- Herrmann HC, Thourani VH, Kodali SK, et al. One-Year Clinical Outcomes With SAPIEN 3 Transcatheter Aortic Valve Replacement in High-Risk and Inoperable Patients With Severe Aortic Stenosis. Circulation 2016;134:130-40. [Crossref] [PubMed]
- Pollari F, Dell'Aquila A, Sohn C, et al. Risk factors for paravalvular leak after transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Agarwal S, Parashar A, Kumbhani DJ, et al. Comparative meta-analysis of balloon-expandable and self-expandable valves for transcatheter aortic valve replacement. Int J Cardiol 2015;197:87-97. [Crossref] [PubMed]
- Barbanti M, Buccheri S, Rodes-Cabau J, et al. Transcatheter aortic valve replacement with new-generation devices: A systematic review and meta-analysis. Int J Cardiol 2017;245:83-9. [Crossref] [PubMed]
- Vogt F, Pfeiffer S, Dell'Aquila AM, et al. Sutureless aortic valve replacement with Perceval bioprosthesis: are there predicting factors for postoperative pacemaker implantation? Interact Cardiovasc Thorac Surg 2016;22:253-8. [Crossref] [PubMed]
- Muneretto C, Bisleri G, Moggi A, et al. Treating the patients in the 'grey-zone' with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg 2015;20:90-5. [Crossref] [PubMed]