
Cardio-toxicity of checkpoint inhibitors
Immune checkpoint inhibitors (ICIs): new challenges for cardio-oncology
The increasing awareness of cardiac side effects of classical oncological therapies has resulted in multiple improvements in cardiac surveillance of cancer patients (1,2). Although classical chemotherapeutic drugs still result in considerable side effects (2), recent studies have shown that cardiovascular mortality is not increased in breast cancer patients any more (3,4). This most probably reflects an increased consideration of cardiac protection when applying chemotherapies, an improved understanding of mechanisms of cardiac damage, and early involvement of cardiologists in following up patients at risk.
Similar to the advances in breast cancer therapy more than two decades ago, a novel class of anticancer drugs has started revolutionizing the treatment of numerous cancers by restoring immune resistance against cancer cells. These so-called ICIs comprise monoclonal antibodies against immune checkpoint molecules. The first substance, ipilimumab, a monoclonal antibody against cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), was introduced for melanoma treatment in 2010 and strikingly improved prognosis of this otherwise fatal disease by doubling the likelihood of long-term survival (5,6). Further antibodies were developed against programmed cell death 1 (PD-1) (nivolumab, pembrolizumab) and its ligand PD-L1 (atezolizumab, durvalumab, avelumab) and successfully applied in multiple solid tumors and hematological malignancies in the last years (7). However, these substances do not only restore antitumor immunity, but may also result in immune-related adverse events (IRAEs) (8,9). These IRAEs were found to be frequent, but responded in most cases well to high doses of steroids (10). Cardiotoxicity was overall low in clinical trials (11). A large safety database from the manufacturer of ipilimumab and nivolumab, Bristol-Myers Squibb, revealed that myocarditis occurred more frequently (0.27% vs. 0.06%) and severely (0.17% vs. <0.01% fatal cases) with combination therapy with ipilimumab and nivolumab compared with single-agent nivolumab (12). Since there were 940 immuno-oncology agents being tested in 3,042 clinical trials in 2017 with a target enrollment of 577,076 patients (13,14), a plethora of new substances are on their way into clinical application. Thus, knowledge of the mechanisms of action, an increasing awareness of potential side effects of ICIs, in particular myocarditis, and strategies to identify patients at risk is mandatory both for oncologists and cardiologists.
How do ICIs restore immunity against cancer cells?
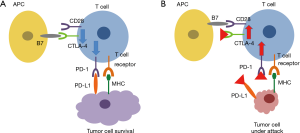
One of the hallmarks of tumor cells is their ability to escape the innate and adaptive immune responses (immunosurveillance) by selection of those cancer cells which are either not immunogenic or are able to suppress an immune response against them (8). T cell activation was initially described as two-signal model in which T cell receptor (TCR) recognition and engagement by major histocompatibility complex (MHC) bound (neo)antigens requires co-stimulatory molecules, CD28 and B7 (CD80/CD86), for an effective immune response (Figure 1) (15). However, several inhibitory pathways, such as CTLA-4 or PD-1/PD-L1 signalling, were identified to efficiently inhibit the antitumor functions of T lymphocytes (8,10,16). CTLA-4 is an immune checkpoint molecule that downregulates T-cell activation by binding to B7 (CD80/CD86) molecules on antigen-presenting cells, opposing CD28-mediated costimulation (Figure 1). Further studies on inhibition of CTLA-4 in preclinical models paved the road to clinical studies investigating this novel mechanism in cancer patients showing an impressive benefit on survival rates in metastatic melanoma patients (5). Similar to CTLA-4 signalling, the PD1/PD-L1 axis was identified as powerful regulator of immunosurveillance. In contrast to CTLA-4, which interacts at the level of the lymph nodes between T-cell and antigen presenting cells, PD-1 and PD-L1 interact at the level of the tumor itself. PD-1 is induced on T cells upon activation and binds to PD-L1 on tumor cells as well as on cells in the proximity of the cancer cell such as local endothelial cells, resulting in suppression of T-cell function (Figure 1). As for CTLA-4, specific blockage of PD-1 and PD-L1 with monoclonal antibodies enables T-cell activation (10).
Cardiac side effects of ICIs
The growing clinical use of ICIs in an increasing number of patients with different cancers was accompanied by publication of individual case reports and case series of patients suffering from severe cardiac side effects (12,17-21). While the initial clinical trials with CTLA4 or PD-1/PD-L1 inhibitors did not indicate cardiac complications, a recent meta-analysis listed 99 cases of cardiac side effects in patients having received ICI (21). Myocarditis was the most frequent presentation (45 out of 99 cases of cardiac toxicity). A total of 27% of the cardiac events were heart failure or cardiomyopathy without signs of myocarditis. Pericardial diseases were reported in 15% of patients with cardiac side effects, conduction diseases in 12% (21). Overall, indications for therapy with ICI was predominantly melanoma (41%), non-small cell lung cancer (26%), and multiple myeloma (9%) (21). Since these cardiac adverse events are rather rare, it is difficult to determine their prevalence. One study published data from 964 patients who received an ICI between November 2013 and July 2017 at Massachusetts General Hospital (20). In total, 1.14% of these patients developed myocarditis and 0.52% developed a major adverse cardiovascular event (MACE) such as death, cardiogenic shock, cardiac arrest, or complete heart block. Interestingly, risk for development of myocarditis was increased in patients with cardiovascular risk factors and combination therapy enhancing anti-tumor effects (14,20). Since ICIs are frequently combined, awareness of ICI-associated myocarditis becomes increasingly important, especially considering that myocarditis occurs rather early: median time to onset of symptoms after first ICI application was 34 days and 81% of cases occurred within 3 months (20).
The role of troponin T as biomarker is quite impressive. Cardiac troponin T was elevated in 26 out of 28 cases (93%) where troponin was measured (21). Moreover, patients who had a higher admission, peak, and final troponin T value had an increased risk for MACE (20). While fatality was high in all patients with cardiac toxicity (35%) and in particular in patients presenting with myocarditis (42%) or complete heart block/conduction disease (55%), prognosis was better in patients with congestive heart failure (death in 26%) or pericardial disease (death in 13%) (21).
Potential mechanisms of immune-mediated myocarditis
ICI-associated myocarditis is characterized by a massive T cell and macrophage infiltration (12,14,17). Although there is currently no clear understanding of the pathophysiology of ICI-associated cardiovascular side effects, basic science data underline a central role of the cellular targets of ICIs in preventing autoimmune myocarditis. For example, deficiency of CTLA-4 in mice is associated with a severe autoimmune myocarditis (22). Furthermore, PD-1 or PD-L1 deficient mice are susceptible to autoimmune myocarditis (23-25). A potential mechanism might be explained by autoantibodies against cardiac proteins like troponin I as observed in PD-1 deficient mice (26). Recent studies showed expression of PD-1 and PD-L1 in human and mouse myocytes as well as a PD-L1 expression on injured cardiomyocytes from patients with ICI-associated myocarditis (12,23,24,27). Since upregulation of both PD-1 and PD-L1 was observed in ischemia/reperfusion after myocardial injury (28), a current hypothesis might be that interaction of PD-L1 with PD-1 protects from the development of autoimmune myocarditis induced by release of cardiac proteins by any kind of cellular damage (e.g., virus infection or toxic effect of drugs). Inhibition of the protective PD-1 and PD-L1 pathway by ICIs might result in myocarditis which further leads to cardiac antigen release and finally a vicious circle (21). In addition, limited data from one patient showed presence of one specific T cell clone in heart, skeletal muscle, and tumor tissue suggesting that these T cells might target an antigen shared by the tumor and skeletal and cardiac muscle (12). Preexisting mechanisms of autoimmunity such as common epitopes or preexisting autoantibodies which are controlled e.g., by PD-1 and PD-L1 might also explain the rapid onset of ICI-associated myocarditis. Further detailed studies in patients suffering from ICI-associated myocarditis are necessary to get insight into the pathomechanism and may include measurement of autoantibodies or characterization of T cell response within heart and tumor.
Diagnosis of immune-mediated myocarditis
The current challenge in diagnosing ICI-associated myocarditis is the lack of clear diagnostic criteria. Myocarditis is defined as inflammation of the myocardium and has a highly variable presentation ranging from elevated biomarkers to different clinical findings such as fatigue, chest pain, heart failure, arrhythmias, heart block, cardiogenic shock, and sudden death (10). Diagnostic pathways should follow guidance provided by recommendations for myocarditis of other causes (29). These include measurement of troponins and electrocardiography (10,14). Echocardiography allows further insights into left-ventricular ejection fraction impairments, wall motion abnormalities, and pericardial effusions. However, a normal echocardiography does not indicate a benign course as in myocarditis of other courses (14,27). Cardiac magnetic resonance imaging enables detection of cardiac edema, fibrosis and necrosis and thus facilitates diagnosis of myocarditis. However, even endomyocardial biopsy as gold-standard for detection of myocarditis is limited in ICI-associated myocarditis as there is frequently a patchy distribution of a lymphocytic infiltrate within or adjacent to regions of myocardial necrosis (14). Therefore, diagnosis of ICI-associated myocarditis might be frequently a diagnosis of exclusion and is based on clinical findings, electrocardiography (EKG), biomarkers (in particular troponins), cardiac imaging, and endomyocardial biopsy. Particular monitoring should be considered for patients with ICI combinations, combinations with other cardiotoxic drugs, preexisting autoimmune diseases or cardiac diseases (10,14). Clinical signs of cardiovascular side effects should result in an immediate cardiological work up (20). In addition, even patients at risk without clinical symptoms have been suggested to be subjected to a pretreatment troponin test and followed up with further tests after 2, 4, and 12 weeks (10) in order to allow an early identification of patients developing an ICI-associated myocarditis.
Therapy of ICI-associated myocarditis
Currently, there are no consensus guidelines for management of patients with ICI-associated myocarditis. It appears wise as subjected by several groups to immediately discontinue ICI therapy in patients with cardiac symptoms and elevated troponins ICI, followed by a thorough cardiological examination (10,14,20). In case a myocarditis is likely or confirmed, high-dose corticosteroid treatment (e.g., methylprednisolone 1 mg/kg) should be initiated (9,10,30), eventually with an initial high dose of 1,000 mg/day for 3 days (10). Although one study has reported lower cardiac complications of myocarditis in patients treated with high doses of steroids (20), this approach remains a reasonable but rather empiric strategy since a systematic review did not find a difference in outcome between patients treated with or without steroids (21). Additional immunosuppressive therapy was suggested for patients with preserved cardiac function (infliximab) or moderate to severe heart failure (anti-thymocyte globulin or tacrolimus) (10).
Conclusions
ICI-associated cardiac adverse reactions are rare but may present as severe myocarditis with variable clinical symptoms. The pathomechanism is incompletely understood and requires further investigation. Especially patients at risk can be followed up by serial troponin measurements in order to allow an early identification of patients developing an ICI-associated myocarditis.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Tilemann LM, Heckmann MB, Katus HA, et al. Cardio-oncology: conflicting priorities of anticancer treatment and cardiovascular outcome. Clin Res Cardiol 2018;107:271-80. [Crossref] [PubMed]
- Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768-801. [Crossref] [PubMed]
- Weberpals J, Jansen L, Muller OJ, et al. Long-term heart-specific mortality among 347 476 breast cancer patients treated with radiotherapy or chemotherapy: a registry-based cohort study. Eur Heart J 2018;39:3896-903. [Crossref] [PubMed]
- Anderson C, Nichols HB, Deal AM, et al. Changes in cardiovascular disease risk and risk factors among women with and without breast cancer. Cancer 2018;124:4512-9. [Crossref] [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889-94. [Crossref] [PubMed]
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol 2017;8:561. [Crossref] [PubMed]
- Varricchi G, Galdiero MR, Marone G, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017;2:e000247. [Crossref] [PubMed]
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [Crossref] [PubMed]
- Wang DY, Okoye GD, Neilan TG, et al. Cardiovascular Toxicities Associated with Cancer Immunotherapies. Curr Cardiol Rep 2017;19:21. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84-91. [Crossref] [PubMed]
- Neilan TG, Rothenberg ML, Amiri-Kordestani L, et al. Myocarditis Associated with Immune Checkpoint Inhibitors: An Expert Consensus on Data Gaps and a Call to Action. Oncologist 2018;23:874-8. [Crossref] [PubMed]
- Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A 1999;96:185-90. [Crossref] [PubMed]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459-65. [Crossref] [PubMed]
- Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. [Crossref] [PubMed]
- Escudier M, Cautela J, Malissen N, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 2017;136:2085-7. [Crossref] [PubMed]
- Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368-76. [PubMed]
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755-64. [Crossref] [PubMed]
- Mir H, Alhussein M, Alrashidi S, et al. Cardiac Complications Associated With Checkpoint Inhibition: A Systematic Review of the Literature in an Important Emerging Area. Can J Cardiol 2018;34:1059-68. [Crossref] [PubMed]
- Love VA, Grabie N, Duramad P, et al. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res 2007;101:248-57. [Crossref] [PubMed]
- Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319-22. [Crossref] [PubMed]
- Lucas JA, Menke J, Rabacal WA, et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol 2008;181:2513-21. [Crossref] [PubMed]
- Wang J, Okazaki IM, Yoshida T, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol 2010;22:443-52. [Crossref] [PubMed]
- Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003;9:1477-83. [Crossref] [PubMed]
- Tocchetti CG, Galdiero MR, Varricchi G. Cardiac Toxicity in Patients Treated With Immune Checkpoint Inhibitors: It Is Now Time for Cardio-Immuno-Oncology. J Am Coll Cardiol 2018;71:1765-7. [Crossref] [PubMed]
- Baban B, Liu JY, Qin X, et al. Upregulation of Programmed Death-1 and Its Ligand in Cardiac Injury Models: Interaction with GADD153. PLoS One 2015;10:e0124059. [Crossref] [PubMed]
- Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-48, 2648a-2648d.
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]


