
Refined risk stratification for thoracoscopic lobectomy or segmentectomy
Introduction
Accurate risk assessment prior to major lung resection is crucial for determining an optimal treatment strategy for patients with early-stage non-small cell lung cancer (NSCLC). The latest guideline for preoperative physiologic assessment was published by the American College of Chest Physicians in 2013 (1). It is noteworthy that the evidence used in this guideline is predominantly based on clinical studies published in the last decade. More importantly, none of those studies specifically focused on patients undergoing thoracoscopic lobectomy, which has been widely adopted in this decade and is currently the preferred surgical approach for treatment of early stage NSCLC (2).
Owing to less trauma to the chest wall and better preservation of lung function in the initial postoperative period, thoracoscopic lobectomy is associated with reduced postoperative mortality and morbidity compared with lobectomy via thoracotomy (3). Cumulative evidence has demonstrated that these benefits over open approach is most evident in high-risk patients, e.g., those with compromised pulmonary function or older age (4,5). Our previous studies also indicated that increased age and comorbidity burden were not associated with higher risk of morbidity following thoracoscopic lobectomy (6,7). Regarding the positive effect of the thoracoscopic approach on improving postoperative outcomes, important questions have arisen as to the recommendation for preoperative physiologic evaluation in the current guideline, which is applicable only to major lung resections via thoracotomy (1). Moreover, the previously published data derived from the large clinical thoracic surgery database showed that the rates of postoperative major complications and overall morbidity were still not negligible, when major lung resection was performed via thoracoscopic approach (5). Accordingly, there is growing interest in refining risk assessment algorithms, specifically for patients undergoing thoracoscopic lobectomy. Thus, we decided to review our institutional database on thoracoscopic major lung resections. The purpose of the current study was to explore the relationships of established predictors to postoperative complications following thoracoscopic lobectomy or segmentectomy and examine their causal effect.
Methods
A retrospective study was performed using our institutional database on thoracoscopic major lung resections that included retrospective data from 2009 to 2014 and prospective data thereafter. From January 2009 through May 2017, 644 patients underwent thoracoscopic lobectomy or segmentectomy at our institution. Of those patients, 606 with confirmed or suspected early-stage NSCLC or pulmonary metastasis were included in the present study. Thirty-eight patients with infectious disease, e.g., bronchiectasis, were excluded, because they comparatively exposed for much higher risk of postoperative complication. This study was approved by the local Institutional Review Board (project number 086/2018BO2), and specific patient consent was waived.
All patients with confirmed or suspected early-stage NSCLC underwent computed tomography, positron emission tomography and brain magnetic resonance imaging for clinical staging. Patients with suspected mediastinal nodal metastases were submitted to endobronchial ultrasound guided fine needle aspiration or cervical mediastinoscopy. All cases were preoperatively reviewed by our lung oncology multidisciplinary team. Lung cancer staging was performed according to the American Joint Committee on Cancer 7th edition manual (8). Pathologic stage was reported based on the final histopathologic findings after lung resection and systematic mediastinal lymph node dissection.
Physiologic evaluation was performed for all patients prior to surgery. The predicted postoperative values of forced expiratory volume in the first second expressed as the percent predicted (ppoFEV1%) and of diffusing capacity of the lung expressed as the percent predicted (ppoDLCO%) were calculated using the functional segment technique (1). Patients in whom preoperative lung function testing demonstrating impaired pulmonary function (ppoFEV1% or ppoDLCO% <40) were subjected to a stair climb test or cardiopulmonary exercise testing (cycle ergometry) for additional risk stratification prior to lung resection. When performance in the stair climbing test was not satisfactory, cycle ergometry was used to determine eligibility. Maximum oxygen consumption >10 mL/kg/min or 35% predicted was considered to be sufficient for warranting major lung resection.
Performance status was considered marginal or poor when the Eastern Cooperative Oncology Group (ECOG) scale score was ≥2. An American Society of Anesthesiologists physical status (ASA-PS) scale score was assigned to all patients by the responsible anesthesiologists prior to surgery according to the modern version (9). In accordance with previous studies, we collapsed multiple ASA-PS grades into high- and low-grade strata, with 1 or 2 representing low grade and 3 or 4 denoting high grade (10,11).
Thoracoscopic lobectomy and segmentectomy were performed using a three-port approach, including a 3-cm anterolateral access incision in the fourth intercostal space without rib spreading and with visualization only through the monitor. Lobar vessels and the bronchus were individually divided. Hilar and mediastinal lymph nodes were dissected.
Mortality was defined as death during hospitalization for surgery or within 30 days of the operation. Postoperative complications were defined according to the Society of Thoracic Surgeons and The European Society of Thoracic Surgeons (STS/ESTS) joint standardization of variable definitions and terminology (12). Pulmonary complications included pneumonia, atelectasis requiring bronchoscopy, adult respiratory distress syndrome, initial ventilator support for >48 hours, unplanned re-intubation, or tracheotomy. Cardiovascular complications were defined as acute myocardial infarction, pulmonary embolism, and atrial or ventricular arrhythmia requiring intervention. Other complications included bronchopleural fistula, empyema, wound infection, other postoperative infections, cerebrovascular events, delirium, renal failure, postoperative bleeding, chylothorax, recurrent laryngeal nerve injury and other relevant events. Overall morbidity was defined as the occurrence of any of the abovementioned complications, including mortality, except for technical complications such as postoperative bleeding, chylothorax and recurrent laryngeal nerve injury.
Categorical variables are expressed as percentages and were evaluated with Fisher’s exact test. Continuous data are reported as the median and interquartile range (IQR) and were compared using the Mann-Whitney test. Logistic regression analyses were performed to assess the relationships of the established risk factors for cardiopulmonary complications and overall morbidity. The following variables were included: gender (male), ppoFEV1%, ppoDLCO%, smoking history, ECOG performance status (≥2 vs. <2), ASA-PS scale score (high grade vs. low grade) and advanced pathologic stage (>II). While ppoFEV1% and ppoDLCO% were modeled as continuous variables, the remaining variables were modeled as binary categorical variables. Age was not included in the logistic regression model, as chronologic age is not considered an absolute risk factor according to the current guideline for physiologic evaluation prior to major lung resection (1). In fact, an accumulating body of published data including our previously published study has illustrated comparable postoperative outcomes between elderly patients and their younger counterparts undergoing major lung resection by either thoracotomy or thoracoscopic approach (7,13). We used two-sample comparisons of proportions to calculate the sample size for logistic regression. It was assumed that postoperative complications occurred in 10% and 20% of patients with low and high risk, respectively (odds ratio =2.25). Accordingly, we estimated that 275 patients were required for each group to reach a statistical power of 90% at a 5% significance level. To yield an accurate estimation of the impact of high-grade ASA-PS, propensity score adjustment using inverse probability of treatment weighting was performed. In this method, a weight was calculated based on propensity score for each subject that was equal to the inverse of the estimated probability of receiving the treatment (high-grade ASA-PS) that was actually received, conditionally on the observed covariates. These weights were then incorporated into a logistic regression model to estimate the effect of high-grade ASA-PS on the outcome variables (14). Statistical significance was declared for P<0.05. Inverse probability of treatment weighting using propensity scores was conducted using R Project for Statistical Computing, version 3.3.3, along with R package “twang” 1.5 and “survey” 3.32 (15-17). The other statistical analyses were performed using SPSS, version 22.0, for Windows (SPSS, Chicago, IL, USA).
Results
This study included 275 women and 331 men with a median age of 67 (IQR, 59–74) years. A total of 252 (41.6%) patients were older than 70 years. The surgical procedures included 544 (89.8%) lobectomies and 62 (10.2%) segmentectomies. In 23 (3.8%) patients, thoracoscopic procedures had to be converted to thoracotomy. Among the entire cohort, pathological analyses demonstrated confirmed NSCLC in 542 (89.4%) patients and pulmonary metastasis in 30 (5.0%) patients, while benign processes were found on pathologic examination in 34 (5.6%) patients with preoperative suspicion for lung cancer. Patient demographics and clinical characteristics are shown in Table 1. Impaired pulmonary function (ppoFEV1% or ppoDLCO% <40) was found in 116 (19.1%) patients. Of these patients, 50 underwent cycle ergometry, with a measurement of the maximum oxygen consumption, ranging from 10.9 to 27.0 mL/kg/min (43.0–162.0%).
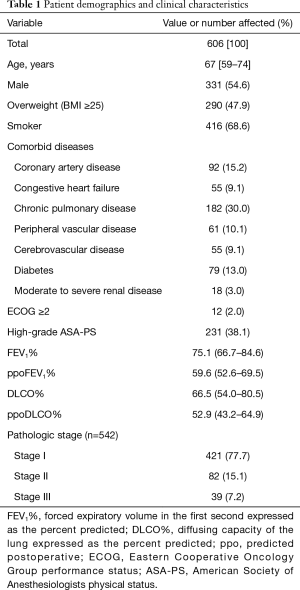
Full table
There were 6 (1.0%) postoperative deaths for the entire patient cohort. Four patients died from postoperative pneumonia. One patient developed a bronchopleural fistula followed by sepsis and multiple organ failure and died on postoperative day 12. Another developed pneumonia and atrial arrhythmia on postoperative day 5 followed by a bronchopleural fistula, and died on postoperative day 79. The postoperative cardiopulmonary complication and overall morbidity occurred in 76 (12.5%) and 109 (18.0%) patients, respectively. The incidences of single postoperative complication are depicted in Table 2.
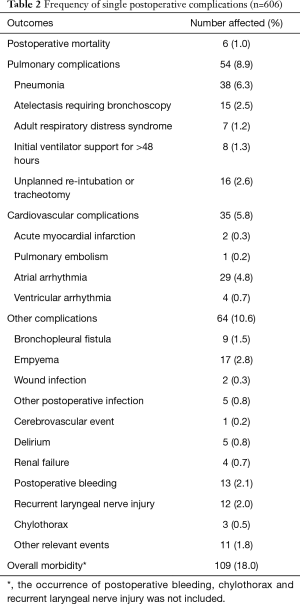
Full table
Table 3 describes the relationships of the established risk factors to the postoperative complications. In the univariable logistic regression analyses, cardiopulmonary complication rates and overall morbidity were not related to male gender, smoking history, advanced pathologic stage or worse performance status (ECOG ≥2). There was a slightly significant relationship of ppoFEV1% and ppoDLCO% with overall morbidity. In contrast, there were specific correlations of high-grade ASA-PS with a greater cardiopulmonary complication rate and overall morbidity. In the multivariable regression analysis, high-grade ASA-PS emerged as the single strong predictor of both cardiopulmonary complication rates and overall morbidity. The odds of cardiopulmonary complications and overall morbidity increased by two-fold in patients with high-grade ASA-PS compared with those with low-grade ASA-PS.
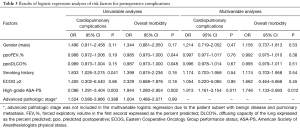
Full table
To examine the causal effect of a high-grade ASA-PS on postoperative adverse outcomes, inverse probability of treatment weighting using propensity scores was performed. In our patient cohort, there were 375 (61.9%) patients with low-grade ASA-PS, with the remainder of patients having high-grade ASA-PS (n=231, 38.1%), including two patients with grade 4. Compared with patients with low-grade ASA-PS, those with high-grade ASA-PS were older and had lower pulmonary function. They were also more likely to be male and to have a history of smoking and worse performance status (Table 4). However, they were less likely to have benign disease. According to these differences in demographics and clinical characteristics, propensity scores were assessed using a multivariable logistic regression model, which included age, male gender, history of smoking, ppoFEV1%, ppoDLCO%, ECOG score (≥2 vs. <2) and benign disease. Inverse probability of treatment weighting using the estimated propensity score resulted in well balanced patient characteristics in both groups except for still significant difference in age (Table 4). Our analysis of weighted data using generalized linear model revealed 2- and 1.7-fold increases in the odds of cardiopulmonary complications and overall morbidity, respectively, in patients with high-grade ASA-PS compared with those with low-grade ASA-PS (odds ratio =2.116, 95% CI: 1.252–3.577, P=0.005; odds ratio =1.740, 95% CI: 1.095–2.765, P=0.019, respectively).
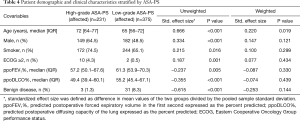
Full table
Discussion
Because of its minimally invasive nature, thoracoscopic lobectomy is associated with less pain and better preservation of lung function in the initial postoperative period (18,19). These advantages, in turn, translate into reduced postoperative adverse events compared with lobectomy via thoracotomy (3). In the present study, thoracoscopic lobectomy resulted in low mortality and reasonable morbidity. Our postoperative outcomes are in accordance with those of the previously published study based on large clinical thoracic surgery database (5). Nevertheless, the rates of major complications and overall morbidity following thoracoscopic lobectomy and segmentectomy were not negligible in our patient cohort despite thorough preoperative physiologic evaluation. This fact implies a greater need for refining preoperative risk stratification in this special patient population.
Lung function determinants, including FEV1% and DLCO%, are essential for the risk stratification prior to major lung resection (1). Due to the consideration of resected functioning lung, ppoFEV1% and ppoDLCO% are admittedly more accurate in predicting postoperative complications than the preoperative values (1). Previous studies suggested that ppoFEV1% and ppoDLCO% are predictive of cardiopulmonary complications regardless of whether lobectomy is performed by an open or thoracoscopic approach (5,20). However, there were only slightly significant relationships of ppoFEV1% and ppoDLCO% with overall morbidity in our univariable analyses. The multivariable regression analyses failed to associate ppoFEV1% and ppoDLCO% with cardiopulmonary complications or overall morbidity after thoracoscopic lobectomy in our study. These results may be explained by our thorough workup and careful patient selection performed before surgery. Indeed, the vast majority of our patients presented with a good preoperative performance status (ECOG score <2). Moreover, in accordance with the guidelines for preoperative physiologic evaluation, all patients with impaired pulmonary function were submitted to a stair climbing test or cardiopulmonary exercise testing for additional risk stratification. Only those patients who presented with satisfactory performance in the stair climbing test or who had adequate maximum oxygen consumption were operated on and included in the present study. In this aspect, our favorable postoperative outcomes indicate that the current physiologic evaluation algorithm is also applicable to identify patients amenable to major lung resection performed via thoracoscopic approach.
Interestingly, the ASA-PS exhibited distinct power in predicting the major complications following thoracoscopic lobectomy in our properly selected patient cohort. Compared with patients with low-grade ASA-PS, those with high-grade ASA-PS had a two-fold greater risk of cardiopulmonary complications and overall morbidity in the multivariable regression analyses. More importantly, causal effect analyses using inverse probability of treatment weighting demonstrated a similar effect size of high-grade ASA-PS. As a currently popular method for causal inference, propensity score matching combined with logistic regression models with random effects was initially considered to analyze the causal effect of high-grade ASA-PS. However, our attempt to perform propensity score matching without replacement for balancing the differences in baseline characteristics between patients with high- and low-grade ASA-PS was unsuccessful because the propensity score distribution was very different in the two groups. While propensity score matching with replacement yielded two groups with well-balanced baseline characteristics, there was reasonable doubt about the results based on the matched data due to a considerably reduced sample size. In contrast, the inverse probability of treatment weighting using propensity scores retained all subjects in the analysis and was believed to be a superior approach for causal effect analyses in our study (14).
The ASA-PS is a classification system that assesses a patient’s overall health status and degree of sickness based on systemic disease. Although it has been widely used by anesthesiologists to estimate operative and anesthesiological risks, the ASA-PS has rarely been considered in the risk assessment process prior to major lung resection to date (21). This is thought to be due, at least in part, to the fact that very few studies have previously investigated the impact of the ASA-PS as a predictor of postoperative adverse outcomes following major lung resection (22,23). Notably, these data are characterized by various limitations, particularly smaller sample sizes. Additionally, little is known about the predictive capability of ASA-PS in patients undergoing major lung resection via minimally invasive approach. In a recent study based on data derived from the STS General Thoracic Database, Fernandez and colleagues evaluated postoperative outcomes of over 27 thousand lung resections through an open or minimally invasive approach (10). They found a 1.25-fold increase in the odds of major complications in patients with ASA-PS grade 3 compared with those with ASA-PS grade 1 or 2. In a study involving 176 elderly patients undergoing thoracoscopic lobectomy, our group has previously reported a nearly 3-fold increase in the odds of cardiopulmonary complications in those with ASA-PS grade =3 compared with patients with ASA-PS grade <3 (7). It is worth mentioning that this finding was based on the univariable logistic regression analyses restricted to elderly patients. In contrast, the present study used multivariable regression model for confounder adjustment and showed that ASA-PS was an independent morbidity predictor in a general patient population considered for thoracoscopic lobectomy or segmentectomy. Moreover, the present study is the first to determine the size of causal effect of high-grade ASA-PS on postoperative complications following major lung resection using propensity-adjusted analysis. Based on our findings, early involvement of anesthesiologists and assignment of an ASA-PS scale are recommended for the risk assessment prior to thoracoscopic lobectomy or segmentectomy. We believe that the ASA-PS, as an easily assessable gauge of surgical risks, can be used to identify patients who require more comprehensive physiologic evaluation prior to major lung resection. In conjunction with other predictors, the ASA-PS will enhance the current algorithms for risk stratification and improve informed decision making for patients with early-stage NSCLC. Due to the possible inter-rater inconsistency, especially in assigning grades 2 (mild systemic disease) and 3 (severe systemic disease), patients with ASA-PS grade 2 or higher should consult with senior anesthesiologists as early as possible (24).
The authors acknowledge potential limitations of the present study. First, there was the inherent bias due to the use of retrospective data. Only thoracoscopic major lung resections in and after 2015 at our institution were prospectively evaluated. Therefore, it is possible that some postoperative adverse events were undisclosed in patients operated on from 2009 through 2014. Additionally, our results are derived from single institution data, which may not fully reflect the clinical scenario elsewhere. On the other side, our postoperative outcomes are in line with those of previously published large-scale studies on thoracoscopic lobectomy (5). Though further investigation involving a multicenter design is necessary to validate our findings, our data may have mitigated the bias caused by the variety in surgical techniques and perioperative care strategies commonly seen in multicenter studies. Selection bias was another concern in the present study. Although propensity score adjustment using inverse probability of treatment weighting mitigated the selection bias to some extent by balancing the known confounders, there may be some unknown confounding variables that influenced the results.
In conclusion, our results suggested that the current physiologic evaluation algorithm is also applicable to identify patients amenable to major lung resection performed by thoracoscopic approach. Despite thorough physiologic evaluation in compliance with the current guideline, the major complications following thoracoscopic lobectomy were still not negligible. As an easily assessable factor the ASA-PS is capable of predicting postoperative adverse events after thoracoscopic lobectomy in properly selected patients. Early involvement of anesthesiologists in the preoperative risk assessment and assignment of the ASA-PS scale are recommended for refined risk stratification in this patient population. Future efforts are warranted to incorporate the ASA-PS into the existing algorithm for risk assessment prior to major lung resection.
Acknowledgements
We acknowledge the substantial contributions of all surgical and anesthesiological team members. We also thank Mr. Sebastian Ciupa for his kind support during data collection.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the local Institutional Review Board (project number 086/2018BO2), and specific patient consent was waived.
References
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Zhang R, Ferguson MK. Video-Assisted versus Open Lobectomy in Patients with Compromised Lung Function: A Literature Review and Meta-Analysis. PLoS One 2015;10:e0124512. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28. [Crossref] [PubMed]
- Zhang R, Kyriss T, Dippon J, et al. Impact of comorbidity burden on morbidity following thoracoscopic lobectomy: A propensity-matched analysis. J Thorac Dis 2018;10:1806-14. [Crossref] [PubMed]
- Zhang R, Kyriss T, Dippon J, et al. American Society of Anesthesiologists physical status facilitates risk stratification of elderly patients undergoing thoracoscopic lobectomy. Eur J Cardiothorac Surg 2018;53:973-9. [Crossref] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. American Joint Committee on Cancer. 7th ed. New York: Springer, 2010.
- American Society of Anesthesiologists. ASA Physical Status Classification System. Schaumburg, IL: American Society of Anesthesiologists; 2014 Oct 15 [cited 2018 Oct 15]. Available online: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370-7. [Crossref] [PubMed]
- Miller TJ, Jeong HS, Davis K, et al. Evaluation of the American Society of Anesthesiologists Physical Status classification system in risk assessment for plastic and reconstructive surgery patients. Aesthet Surg J 2014;34:448-56. [Crossref] [PubMed]
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5. [Crossref] [PubMed]
- Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health 2006;60:578-86. [Crossref] [PubMed]
- Ridgeway G, McCaffrey D, Morral A, et al. twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. R package version 1.5. 2017 Jul 2 [cited 2018 Oct 15]. Available online: https://CRAN.R-project.org/package=twang
- Lumley T. Analysis of Complex Survey Samples. J Stat Soft 2004;9:1-19. [Crossref]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [cited 2018 Oct 15] Available online: https://www.R-project.org
- Endoh H, Tanaka S, Yajima T, et al. Pulmonary function after pulmonary resection by posterior thoracotomy, anterior thoracotomy or video-assisted surgery. Eur J Cardiothorac Surg 2010;37:1209-14. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Zhang R, Lee SM, Wigfield C, et al. Lung function predicts pulmonary complications regardless of the surgical approach. Ann Thorac Surg 2015;99:1761-7. [Crossref] [PubMed]
- Hackett NJ, De Oliveira GS, Jain UK, et al. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg 2015;18:184-90. [Crossref] [PubMed]
- Ciszewski P, Tyczka J, Nadolski J, et al. Lower preoperative fluctuation of heart rate variability is an independent risk factor for postoperative atrial fibrillation in patients undergoing major pulmonary resection. Interact Cardiovasc Thorac Surg 2013;17:680-6. [Crossref] [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: Are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Riley R, Holman C, Fletcher D. Inter-rater reliability of the ASA physical status classification in a sample of anaesthetists in Western Australia. Anaesth Intensive Care 2014;42:614-8. [Crossref] [PubMed]


