
Da Vinci Xi robot decreases the number of thoracotomy cases in pulmonary resection
Introduction
Minimally invasive pulmonary resection has been shown to have numerous benefits over pulmonary resection via a thoracotomy. Patients have significantly less morbidity and length of stay with video-assisted thoracoscopic surgery (VATS) compared to open thoracotomy (1-3). An analysis comparing robot-assisted surgery without the vascular stapler and open thoracotomy shows significant improvement for patients who underwent robot-assisted surgery compared to an open thoracotomy (4). The robot without a vascular stapler (da Vinci Si®, Intuitive Surgical, Sunnyvale, CA, USA) has provided surgeons with increased dexterity and improved visualization in the operative field. However, the lack of the vascular stapler has led to the reliance on a bedside assistant to help with critical parts of the surgical procedure. The addition of the robot with the vascular stapler has dramatically improved surgeon autonomy during pulmonary resections. The technological improvement of adding a vascular stapler may lead to significant improvement in the outcomes of the robot surgery compared to the VATS procedure. In addition, improved autonomy and increased dexterity and visualization has improved surgeons’ ability to perform more complex pulmonary resection using minimally invasive techniques (5). We wanted to determine if the addition of a vascular stapler (da Vinci Xi®, Intuitive Surgical, Sunnyvale, CA, USA) decreases the number of patients requiring a thoracotomy, both with cases electively performed with a thoracotomy or with cases converted to a thoracotomy from a planned minimally invasive approach.
Methods
The study was approved by the Institutional Review Board at the Houston Methodist Research Institute. We reviewed all patients who underwent pulmonary resection at Houston Methodist Hospital performed by surgeons in the Division of Thoracic Surgery from 2012 to 2017. We excluded all patients who underwent diagnostic wedge resections, emergent pulmonary resection and patients who underwent pulmonary resection as part of a “two-step” procedure for cardiac sarcoma with pulmonary involvement (6). We evaluated patient demographics, clinicopathologic features, surgical information and surgical outcomes obtained from prospectively collected Society of Thoracic Surgeons (STS) data at Houston Methodist Hospital. We evaluated the impact of the addition of the robot with vascular stapler (da Vinci Xi®) on the number of cases that required thoracotomy. We divided the patients into two time periods: prior to (pre-robot) and after (post-robot) the introduction of the robot with a vascular stapler (da Vinci Xi®). We also analyzed the number of years after completion of training for the surgeons in the practice. Surgical experience was categorized as ≤5 years from the end of fellowship training and >5 years from the end of fellowship training during the two-time periods.
Demographic and clinical data were reported as frequencies and proportions for categorical variables and as medians and interquartile ranges (IQR) or means (±standard deviation, SD) for continuous variables as appropriate. The difference between the groups was compared using the Chi-square or Fisher’s exact tests for categorical variables and unpaired t-test or Kruskal Wallis test for continuous variables as appropriate. Univariate and multiple logistic regression analyses were performed to determine the characteristics associated with various outcomes. Variables having a P value of <0.2 in the univariate analysis and variables considered as clinically important were then investigated further by multiple logistic regression. Variables for multiple logistic regression models were then selected using the Bayesian model averaging (BMA) method to include the variables with a high probability of being a risk factor (7,8). Stata (StataCorp LLC, College Station, TX, USA) BMA program was run to evaluate possible model sets. The Likelihood Ratio test was used to further reduce the model subsets. The best model was selected based on the smallest Bayesian information criterion (BIC). A P value <0.05 was considered statistically significant.
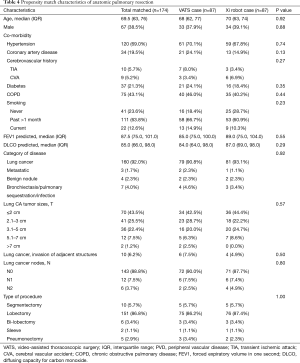
We evaluated VATS compared to the robot with a vascular stapler for anatomic resection in terms of surgical outcomes, especially conversions to open thoracotomy. In order to compare the outcomes between VATS and robotic pulmonary resection, we conducted a one-to-one propensity score matching. The propensity score was estimated based on a set of covariates including age, gender, race, height, weight, Zubrod score, American Society of Anesthesiologists (ASA) classification, category of disease, type of procedure, forced expiratory volume in 1 second (FEV1), carbon monoxide lung diffusion capacity (DLCO), and, in the case of lung cancer, lung cancer pathologic tumor size (T), lung cancer nodules (N) and presence of metastasis (M). All of the analyses and propensity score matching were performed using Stata version 14.2 (StataCorp LLC, College Station, TX, USA). A P value of <0.05 was considered statistically significant.
Results
A total of 389 patients met the inclusion criteria from 2012–2017. The robot with vascular stapler (da Vinci Xi®) was obtained in January 2016 at Houston Methodist Hospital and the first patient who had pulmonary resection with this robot underwent surgery in February 2016. During the pre-robot time period, there were 220 cases (56.6%), of which 19 cases (8.6%) started with a thoracotomy, 7 cases (3.2%) were performed minimally invasively with the robot without the vascular stapler (da Vinci Si®) and 194 cases (88.2%) were performed with a VATS. During the post-robot time period, there were 169 cases (43.4%), of which 2 cases (1.2%) were performed with an open thoracotomy, 118 cases (69.8%) were performed with the robot with the vascular stapler (da Vinci Xi®), and 49 cases (29%) were performed with a VATS (Figure 1).
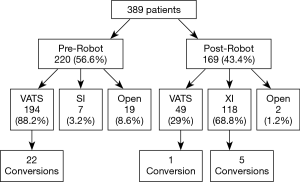
There were a total of four different surgeons during the study period; however, there were three surgeons during each time period with similar experience. During the pre-robot period, one surgeon had finished training >5 years from the start of the time period and two surgeons were ≤5 years from finishing training. During the post-robot period, the most senior surgeon left the practice which left one surgeon who had finished training >5 years at the start of the time period and two surgeons who were ≤5 years from finishing their training. One senior and one junior surgeon during the post-robot period had used the robot without a stapler (da Vinci Si®) for select cases in practice. The other junior surgeon had never used the robot in fellowship training or at the start of the post-robot period. Moreover, assistants for the VATS and da Vinci Xi® were general surgery residents either post graduate level 1, 3 or 4 who had about 1 to 2 months of thoracic surgery experience while for da Vinci Si®, attending surgeon assisted with the case.
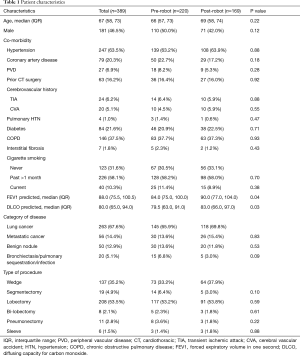
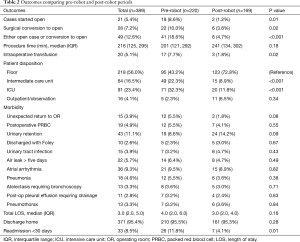
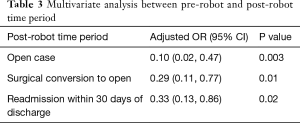
Overall, the patients’ median age at the time of surgery was 67 years old. Most of the patients were female (208, 53.5%) and white (309, 80.1%) with a median body mass index (BMI) of 27.1. There were no significant differences between the pre-robot and post-robot group in co-morbidities, Zubrod score and ASA classification (Table 1). The median FEV1 predicted (84 vs. 90, P=0.04) and DLCO predicted (79.5 vs. 83, P=0.03) were significantly lower in the pre-robot group than the post-robot group. The patients’ final pathology showed lung cancer in 263 (67.6%), metastatic lung cancer in 56 (14.4%) and benign lung nodules in 50 (12.9%). Twenty patients (5.1%) had bronchiectasis, infection. Univariate analysis showed that in the pre-robot period, significantly more cases started with an open thoracotomy (8.6% vs. 1.2% P=0.01), more cases converted from minimally invasive technique to open technique (10% vs. 3.6%, P=0.02) and more patients who ended up with open thoracotomy (18.6% vs. 4.7%, P<0.001). Moreover, in the pre-robot period, more patients received blood transfusions (7.7% vs. 1.3%, P=0.02) and went to an intermediate care unit (22.3% vs. 8.9%, P<0.001) or intensive care unit (ICU, 32.3% vs. 11.8%, P<0.001) rather than to a regular surgical floor. There was no difference in other morbidities, mortality and length of stay after the operation. However, there were significantly more readmissions within 30 days in the pre-robot time period compared to the post-robot time period (11.8% vs. 4.1%, P=0.01, Table 2). Multivariate analysis showed that the post-robot time period had significantly fewer open cases (P=0.003), fewer conversions to open thoracotomy (P=0.01), and fewer readmissions within 30 days of discharge (P=0.02, Table 3).
Full table
Full table
Full table
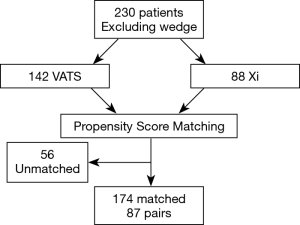
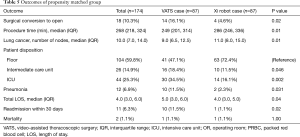
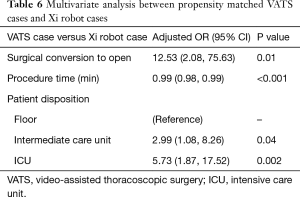
Next, we performed propensity matching between the patients who underwent anatomic resection with VATS (n=87) to patients who underwent surgery with the robot with vascular stapler (n=87, da Vinci Xi®) and evaluated the outcomes (Figure 2, Table 4). In this matched group, there were significantly more patients who had conversion to open thoracotomy (16.1% vs. 4.6%, P=0.02, Table 5), significantly less procedure time (249 vs. 286 minutes, P=0.01), significantly fewer total number of lymph nodes resected (9 vs. 11, P=0.01), more patients who went to the intermediate care unit (18.4% vs. 11.5%, P=0.046), more patients who went to the ICU (34.5% vs. 16.1%, P=0.002), a greater median length of stay (5 days vs. 4, P=0.04), and a higher frequency of 30 day readmissions (11.5% vs. 1.1%, P=0.031) in the VATS group compared to the robot group. There was reduction of number of conversion due to anatomy with da Vinci Xi® compared to VATS. The reasons for 30-day readmission for the VATS group were pneumothorax, pleural effusion, pneumonia, bronchopleural fistula and colonic distension while the reason for readmission for the da Vinci Xi® group was pulmonary emobli. There was no significant difference in morbidity and 30-day mortality between the groups. The multivariate analysis showed that the VATS procedure had more conversions to open thoracotomy [OR =12.5 (2.08–75.63), P=0.01], shorter procedure time [OR =0.99 (0.98–0.99), P<0.001], more patients who went to an intermediate care unit [OR =2.99 (1.08–8.26), P=0.04], and more who went to ICU [OR 5.73 (1.87–17.52), P=0.002, Table 6].
Full table
Full table
Full table
Conclusions
In evaluating a patient for pulmonary resection, the thoracic surgeon makes a decision regarding the optimal surgical approach to successfully perform the operation. The decision is based on imaging criteria from a computed tomography (CT) scan, the surgeon’s level of skill and experience, and the tools that are available to the surgeon. Open thoracotomy was the standard of care until the VATS techniques were developed in the early 1990s. Minimally invasive techniques with new instruments have enabled the surgeons to provide the same operation with significantly less morbidity. Patients who had VATS lung resection went home earlier compared with patients who underwent open thoracotomy while experiencing less pain and fewer complications (3). However, certain anatomic findings seen on the CT scan, such as large tumor size, incomplete fissure and tumors close to the hilum, often precluded surgeons from attempting or successfully completing a VATS procedure (9,10). Some of these limitations have been overcome with new surgical tools (11). The robot with a vascular stapler (da Vinci Xi®) is a significant improvement over the previous robotic platform and allows for better surgeon control during the operation, especially stapling of the vascular structures (5,12). Our study examined the value of having access to a robot with the vascular stapler (da Vinci Xi®) in a general thoracic surgery program. We found that after having (and training with) a robot with a vascular stapler, we had a significant decrease in the percentage of patients requiring an open thoracotomy from 18.6% to 4.7%. Since minimally invasive procedures have shown to decrease overall pain, length of stay and morbidity compared to open thoracotomy in other studies with larger number of patients (13,14) the decrease in thoracotomy rate will improve the overall outcome for patients requiring lung resection over time. The propensity-matched analysis comparing the VATS with robotic approach has shown significantly fewer conversions to thoracotomy in the robot group. In other studies, the conversion rate from VATS to thoracotomy is as high as 28% with a 35% rate of planned thoracotomy, resulting in a combined thoracotomy rate of 63% (15). Thus, our combined conversion and planned thoracotomy rate of 4.7% provides a significant benefit to our lung resection patients.
Our finding is consistent with other propensity-matched studies using the Premier database comparing VATS to robotic approaches which also demonstrated a significant decrease in conversion rate with the robot procedure (16). Unfortunately, the Premier data (16) does not provide granular details about the individual surgeons performing the operation. Our study uses information about the number of years post fellowship completion as a marker for experience. Studies have shown that the skill and experience of the surgeon in performing VATS lobectomy correlates with the number of cases that were converted to thoracotomy. At Washington University School of Medicine, as the surgeons gained experience with VATS pulmonary resection, conversions to thoracotomy dropped significantly from 28% to 11% during their study period (17). In our study, there were two junior surgeons and one senior surgeon in the practice in each time period. Thus, one would expect the conversion rate to thoracotomy to be high during both time periods. In addition, all of the surgeons in the practice were early in the learning curve of using the robot for pulmonary resection after acquisition of the robot, which would be expected to correlate with an increased conversion rate to thoracotomy. Initially, we did not perform robot assisted lung resection in a standardized procedure, but after several months we quickly adopted a “five on a dice” port placement and technique (5,12). Thus, in the background of junior surgeons adapting to new technology, we wanted to determine the value of having access to the robot with a vascular stapler. Despite these challenges, significantly fewer patients had a thoracotomy after we had access to the robot with a vascular stapler with significantly more lymph nodes harvested during robot assisted surgery. Moreover, there were fewer readmissions within 30 days after surgery during this time period.
The study’s strength is that due to change in personnel during the study period, there were similar levels of surgical experience in both time periods. Thus, both time periods had a similar level of expertise and experience evaluating each patient. The study’s limitation is that it was a retrospective study evaluating two different time periods and the decision to perform open or minimally invasive surgery was a decided by a surgeon. The decreased number of patients going to the intermediate care unit and intensive care unit during the two time periods may be more of a reflection of full adoption of our enhanced recovery after surgery (ERAS) program (18,19) in 2016 than simply the addition of the robot with a vascular stapler as a surgeon’s tool. Moreover, this is not a randomized control trial between VATS and robot assisted pulmonary resection and thus the patient groups were not exactly the same in the two time periods. However, we performed propensity matching to look at the anatomic pulmonary resection between VATS and robot that still showed significant decrease in thoracotomy rate in the robot pulmonary resection.
Despite these factors, our study shows that providing surgeons with access to a robot with a vascular stapler significantly improves key outcomes after pulmonary resection, higher number of lymph node yield and reducing the number of thoracotomies and 30-day readmissions. The benefits to the patients may be greater as surgeons gain experience using the robot. Further studies are needed to further define the value of the robot in a thoracic surgery practice.
Acknowledgements
We thank Anna Saikin for language editing of the manuscript. We thank Kathryn J. Schulze and Debra Selig-Rosen for providing the data collected for the Society of Thoracic Surgery at Houston Methodist Hospital.
Footnote
Conflicts of Interest: M Kim has consulted for Intuitive Surgical, Olympus, Boston Scientific and Medtronics. Edward Chan has consulted for Olympus, Boston Scientific and Medtronics. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board at the Houston Methodist Research Institute (No. 00013298).
References
- Grogan EL, Jones DR. VATS lobectomy is better than open thoracotomy: what is the evidence for short-term outcomes? Thorac Surg Clin 2008;18:249-58. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Khan N, Fikfak V, Chan EY, et al. "Five on a Dice" Port Placement Allows for Successful Robot-Assisted Left Pneumonectomy. Thorac Cardiovasc Surg Rep 2017;6:e42-4. [Crossref] [PubMed]
- Chan EY, Reul RM, Kim MP, et al. The "Texas Two-Step" procedure. J Thorac Cardiovasc Surg 2018;155:285-7. [Crossref] [PubMed]
- Wasserman L. Bayesian Model Selection and Model Averaging. J Math Psychol 2000;44:92-107. [Crossref] [PubMed]
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993;138:923-36. [Crossref] [PubMed]
- Marty-Ane CH, Canaud L, Solovei L, et al. Video-assisted thoracoscopic lobectomy: an unavoidable trend? A retrospective single-institution series of 410 cases. Interact Cardiovasc Thorac Surg 2013;17:36-43. [Crossref] [PubMed]
- Zhao H, Bu L, Yang F, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer: the learning curve. World J Surg 2010;34:2368-72. [Crossref] [PubMed]
- Nakanishi K. Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. [Crossref] [PubMed]
- Kim MP, Chan EY. "Five on a dice" port placement for robot-assisted thoracoscopic right upper lobectomy using robotic stapler. J Thorac Dis 2017;9:5355-62. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Kim SW, Hong JM, Kim D. What is difficult about doing video-assisted thoracic surgery (VATS)? A retrospective study comparing VATS anatomical resection and conversion to thoracotomy for lung cancer in a university-based hospital. J Thorac Dis 2017;9:3825-31. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61, 62.e1.
- Kim MP, Chan EY, Meisenbach LM, et al. Enhanced recovery after thoracic surgery reduces discharge on highly dependent narcotics. J Thorac Dis 2018;10:984-90. [Crossref] [PubMed]
- Brown JK, Singh K, Dumitru R, et al. The Benefits of Enhanced Recovery After Surgery Programs and Their Application in Cardiothoracic Surgery. Methodist Debakey Cardiovasc J 2018;14:77-88. [PubMed]


