
Dynamics and prognostic value of B-type natriuretic peptide in left ventricular assist device recipients
Introduction
Left ventricular assist device (LVAD) therapy is an established treatment option for advanced heart failure patients, which prolongs survival and improves symptoms and quality of life (1). A steadily growing number of LVAD patients receive prolonged device support, as nearly 50% of LVAD recipients are currently assigned to destination therapy, recovery rates remain low and the availability of donor organs is very limited. Recent advances in device technology and better patient selection has led to significant improvements in long-term outcome. Currently, the estimated one-year survival rate after LVAD implantation is 80%. Nonetheless, LVAD therapy is still inferior to heart transplantation, which remains the gold standard therapy for end-stage heart failure patients. Ultimately half of the patients on device support die from multi-organ failure or cardiovascular causes (2). Thus, better prognostication and accurate risk stratification of this growing patient population are of paramount importance. This can be extremely challenging, as LVAD therapy is accompanied by various physiologic alterations and certain diagnostic tools are restrictedly applicable and do not necessarily reflect the ongoing pathophysiological changes on molecular level. The ability to estimate disease status and predict outcome by measurement of a single biomarker or a panel of biomarkers underlying different pathophysiological processes of the heart failure syndrome may have a crucial therapeutic impact. Previous studies reported a significant decrease in B-type natriuretic peptide (BNP) after LVAD implantation but only scarce data exist regarding the prognostic value of BNP in this patient population (3-10). The purpose of the current study was to investigate the utility of absolute BNP values and their dynamic changes for the prediction of death or the combined end-point of death or hospitalization up to 180 days following LVAD implantation.
Methods
We retrospectively reviewed the database of our interdisciplinary heart failure unit to identify consecutive adult patients who received a continuous-flow LVAD at our institution from December 1, 2010, to June 30, 2016. Clinical data regarding medical history and disease status in the recent preoperative period were prospectively collected in a digitalized database dedicated to clinical surveys. Follow-up data were extracted from the patients’ electronic health records. The follow-up visits in the outpatient clinic were scheduled 1, 2, 3 and 6 months after implantation according to an established internal protocol. Additional visits or inpatient treatments were planed depending on the clinical course and the occurrence of adverse events. The continuous-flow HVAD (HeartWare, Framingham, MA, USA) was the only type of LVAD implanted during this period. The study was approved by the ethics committee of the University of Duisburg-Essen.
Blood samples were collected in EDTA-containing tubes and centrifuged. Plasma was obtained and frozen at −20 °C until analysis. BNP was measured using a commercially available enzyme-linked immunosorbent assay kit. The last available BNP value within two days prior to LVAD implantation was selected as the baseline value and the one measured at the 90-day visit at the outpatient clinic as the follow-up value. In case of a hospitalization at this time point, the next available BNP value measured in the outpatient setting was used instead.
Continuous variables were expressed as means (standard deviations) unless indicated otherwise, and categorical variables as counts (percentages). Continuous data were evaluated for normality of distribution using the Shapiro-Wilk test. For descriptive analysis, the study cohort was categorized to tertiles of baseline BNP. Differences in continuous variables across BNP tertiles were tested with the one-way analysis of variance (ANOVA) or the Kruskal-Wallis H test. The Chi-square test for trend or the Fisher’s exact test were used for testing association between categorical variables and BNP tertiles. A paired-samples t-test was used to determine whether there was a statistically significant difference between the baseline BNP and the 90-day BNP level after log-transformation of the data. The independent predictive value of BNP for the occurrence of all-cause death and the combined end-point of all-cause death or hospitalization was estimated by the Cox proportional hazards regression analysis with a forward variable selection procedure. Variables were introduced in the model based on clinical relevance and if previously tested significant at P<0.10 in the univariate analysis. Kaplan-Meier survival curves were designed to illustrate the survival differences by direction of BNP change from baseline as assessed at 90 days post-implantation. The log-rank test was performed to estimate differences among groups. The level of significance was set to 0.05 and all P values were two-tailed. All analyses were performed using SPSS statistical software (IBM Corp., SPSS Statistics, Version 23.0. Armonk, NY, USA).
Results
Preoperative characteristics of the study population are listed in Table 1. In our cohort median BNP level at baseline was 885 [interquartile range (IQR): 450–1,624] pg/mL, with 99% of measurements above the cut-off of 35 pg/mLfor chronic heart failure and 96% above 100 pg/mL. The mean age was 59 years and nearly 80% of the study patients were males. The majority were inotrope dependent, hospitalized patients in critical or declining clinical status (INTERMACS profile 1–3). Approximately two-thirds of the study population were assigned to destination therapy (67%). Individuals in the highest BNP tertile had more frequently heart failure of non-ischemic etiology (P=0.02) and more severe impairment of cardiac index, (mean cardiac index by ascending BNP tertile: 1.88 vs. 1.86 vs. 1.59 L/min/m2, P=0.03), compared with patients in the lowest tertile. Furthermore, patients in the highest BNP tertile had a higher degree of renal failure, based on the estimated glomerular filtration rate (GFR) (P=0.04). Patients with invasive hemodynamic and respiratory support preoperatively, as well as inotrope-dependent patients, did not exhibit a preponderance in higher BNP tertiles.
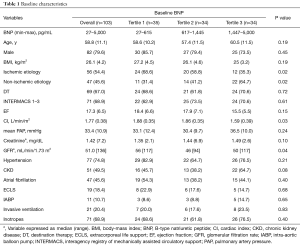
Full table
During the 180-day observational period no device exchanges, device explantations for recovery, or heart transplantations took place and all patients were on continuous LVAD support. There were 43 (41.7%) deaths and 29 (28%) hospitalizations by 180 days, while the combined outcome of death or hospitalization occurred in 69 (67.0%) patients. Higher baseline BNP level was not associated with higher risk of death [hazard ratio (HR) 1.35; 95% CI: 0.92–1.96; P=0.12] or death/hospitalization (HR 1.00; 95% CI: 0.76–1.33; P=0.98) at 180 days (Table 2). Overall 63 (61%) patients survived to 90-day follow-up. The median BNP level at 90-day follow-up was 289 (IQR: 154–534) pg/mL. At the time of BNP sampling no changes were conducted in device function parameters (e.g., device speed). In all subjects, BNP was above the cut-off of 35 pg/mL for chronic heart failure and in 91% above 100 pg/mL. BNP level at 90-day follow-up portended an increased risk for the combination of death or hospitalization up to 180 days post-implantation in the univariate analysis (HR 1.03; 95% CI: 1.03–1.06; P=0.006). After adjusting for relevant covariates, this association did not reach statistical significance (HR 1.01; 95% CI: 0.98–1.04; P=0.62). Table 2 provides an overview of the univariate and multivariate analysis for the study end-points and a list of the variables included in the model.

Full table
At 90 days BNP levels significantly decreased from baseline (median BNP 885 vs. 289 pg/mL, P<0.001). The median absolute change from baseline was −421 (IQR: −1,016 to −46) pg/mL. At 90 days there was no BNP lowering in 21% of patients. This was not associated with worse outcomes, including death, hospitalization or the combination of death/hospitalization at 180 days, when compared with subjects showing any decrease in BNP (Figure 1). Kaplan-Meier curves illustrate the hospitalization-free survival by BNP deviation, i.e., decrease vs. increase/no change (Figure 2, log-rank test: 0.08). As depicted, a non-significant trend towards lower death risk for the group of patients with BNP decrease is seen approximately from day 140 post-implantation.
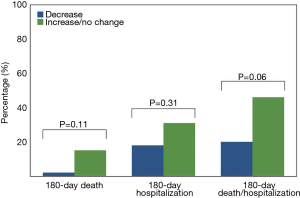
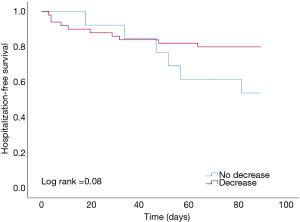
Discussion
BNP is released from ventricular myocardium as a response to volume expansion or pressure overload. It serves an important regulatory role by opposing vasoconstriction, sodium retention and antidiuretic effects of the activated renin-angiotensin-aldosterone system. BNP and the inactive fragment of its precursor molecule, amino-terminal pro-B-type natriuretic peptide (NT-proBNP), are strong predictors of clinical outcomes in patients with all heart failure stages and are the most widely applicable biomarkers in clinical practice (11,12). In our study cohort nearly all patients exhibited BNP values above the cut-off for chronic heart failure at baseline, as defined in the current guidelines (13). This reflects the advanced disease state of LVAD recipients, as well as the clinical profile of our study sample, which consisted mainly of patients in critical or declining disease status at the time of device implantation. Unsurprisingly, patients with higher BNP levels had a significantly lower cardiac index, as well as more severe renal impairment, as estimated by the GFR. As shown in previous studies, patients with chronic kidney disease exhibit elevated BNP levels, which is likely attributed to a multifactorial mechanism rather than a decreased passive renal clearance, but this fact does not significantly influence the prognostic performance of BNP in this subgroup of patients (14). With regard to the preponderance of patients with non-ischemic etiology of heart failure in the higher BNP tertile, it is possibly attributable to the specific profile of the study population and partially to the subgroup of patients with acute myocarditis, who received LVAD as a bridge to recovery due to refractory cardiac decompensation preoperatively.
Following LVAD implantation, a statistically significant reduction in BNP levels was observed at 90 days with a mild to moderate median change of −421 pg/mL. Nonetheless, follow-up BNP levels remained abnormal (>35 pg/mL) in all study patients and above 100 pg/mL in 91% of subjects. Older observational reports have demonstrated that BNP levels in peripheral circulation and BNP mRNA expression in myocardial tissue decrease after LVAD implantation and reach a steady state at approximately 1 to 3 months post-implantation (3-7). These early studies have included small patient samples (n<40) receiving the previous generation pulsatile-flow devices, implanted as a short-term, bridging therapy. Additionally, the BNP changes were not related to long-term clinical outcomes and the magnitude of BNP reduction was not consistently reported. A recent study on 72 continuous-flow LVADs revealed that NT-proBNP levels remained above the upper limit of normal at discharge in all patients and higher absolute levels were significantly associated with a complicated postoperative recovery up to discharge (8). The most comprehensive report on biomarkers in LVAD patients was recently published (9). The authors conducted a prospective study with serial measurements of heart failure biomarkers reflecting distinct pathophysiological pathways in 37 patients. The study demonstrated that NT-proBNP, Galectin-3, copeptin, ST2, growth differentiation factor-15 and C-reactive protein, but not neutrophil gelatinase associated lipocalin, substantially decreased after long-term LVAD support, compared to the preoperative levels, but remained highly abnormal and higher than the previously reported levels in patients with chronic heart failure. Our findings on the BNP dynamics after LVAD placement corroborate the results of these studies and deliver supporting evidence of a persistent dysfunction despite LVAD treatment. Regarding the nowadays universally implemented continuous-flow devices, these data suggest that the macrophysiological improvements achieved by LVAD support do not coincide with mitigation of key pathological changes on the molecular level. The persistent elevation of natriuretic peptides may have several causes. These include device related parameters (low speed setting, inflow obstruction or suction episodes, lack of pulsatility), concomitant valvular disease, the LVAD-associated chronic inflammatory response triggering BNP release, an under-utilization of neurohormonal blockade in LVAD recipients or the fact that continuous-flow devices are associated with less mechanical unloading compared to the pulsatile pumps (15). Consequently, targeted therapeutic interventions have the potential to modify these persisting dysfunctions and may have an influence on clinical outcome or even improve the rates of myocardial recovery after LVAD support.
Serial assessments of BNP were previously shown to provide incremental data regarding the likelihood of an adverse outcome in patients with chronic heart failure (16). Our analysis revealed that absolute BNP values, as measured preoperatively and at 90-day follow-up, failed to predict adverse outcomes at 180 days post-implantation. In a previous study, serial BNP measurements were performed in 83 patients before LVAD implantation and at 30, 60 and 90 days thereafter (10). The authors found that only the BNP levels at 60-days were associated with worse survival, as shown by univariate analysis. However, BNP did not preserve its prognostic accuracy in the multivariate analysis (HR: 1.00). This retrospective study included only 22% continuous-flow devices and performed a longer mean follow-up of 717 days compared to our study. Nearly 80% of the patients in our cohort exhibited a decrease in BNP levels, as measured 90 days after device placement. BNP decrease from baseline was not associated with reduced morbidity and mortality up to 180 days, compared to BNP increase. These results suggest that the predictive value of BNP is abolished in LVAD recipients. There may be several reasons for this. Firstly, LVAD therapy is accompanied by complex cardiovascular and systemic sequelae. The neurohormonal activation and its counter-regulatory pathways, such as those involving natriuretic peptides, may be altered under the continuous, non-pulsatile flow. Second, the current study consisted mainly of high risk, older individuals, 70% of whom were non-eligible for bridging therapies, including a minority who received LVAD as a salvage treatment. Therefore, certain conditions, even of non-cardiac origin such as multi-organ failure, or infections (17), may be the prevailing abnormalities affecting clinical outcomes in our population. Last, it is possible that a single heart failure biomarker may be only weakly associated with major adverse events in LVAD patients. A multimarker approach representing distinct biological pathways and end-organ disease, such as markers of fibrosis, inflammation, renal, hematologic and hepatic function, may be superior to a single biomarker approach in this setting.
The current study has the inherent limitations of a retrospective, single-center study. The sample size was relatively small and we studied one device type. The majority of patients were high-risk individuals, assigned to destination therapy and that is accompanied by an underrepresentation of the bridging therapies. Furthermore, data regarding concomitant medical therapy e.g. neurohormonal blockade, that may have an impact on BNP levels, were not available for analysis. Last, we studied a single biomarker, while NT-proBNP levels and other biomarkers were not consistently measured at follow up and were excluded from analysis. However, this is the first study to investigate the prognostic performance of BNP and its dynamic changes in a population treated exclusively with the new generation continuous-flow devices.
Our study delivers important evidence of ongoing abnormalities triggering BNP release after LVAD implantation. We found that BNP levels significantly decrease, but remain substantially high following device implantation. The predictive value of BNP for the composite of all-cause death or hospitalization in this population is abolished. These findings substantiate the need for further research to identify the mechanisms of disease progression. The complex pathophysiologic alterations following mechanical circulatory support may be better identified and treated by utilization of a multi-marker approach, that integrates diverse pathophysiological pathways.
Acknowledgements
We would like to thank Mr. Klaus Kreikemeier for his contribution to data collection and technical support.
Funding: This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) to TR (Ra969/7-2) and to PL (LU2139/2-1) and the research committee of the University Duisburg-Essen (IFORES research grant) to PL.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The current study was approved by the ethics committee of the University of Duisburg-Essen (17-7923-BO).
References
- Rose EA, Gellins AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [Crossref] [PubMed]
- Kirklin JK, Cantor R, Mohacsi P, et al. First Annual IMACS Report: A global International Society for Heart and Lung Transplantation Registry for Mechanical Circulatory Support. J Heart Lung Transplant 2016;35:407-12. [Crossref] [PubMed]
- Milting H, El Banayosy A, Kassner A, et al. The time course of natriuretic hormones as plasma markers of myocardial recovery in heart transplant candidates during ventricular assist device support reveals differences among device types. J Heart Lung Transplant 2001;20:949-55. [Crossref] [PubMed]
- Sodian R, Loebe M, Schmitt C, et al. Decreased plasma concentration of brain natriuretic peptide as a potential indicator of cardiac recovery in patients supported by mechanical circulatory assist systems. J Am Coll Cardiol 2001;38:1942-9. [Crossref] [PubMed]
- Bruggink AH, de Jonge N, van Oosterhout MF, et al. Brain natriuretic peptide is produced both by cardiomyocytes and cells infiltrating the heart in patients with severe heart failure supported by a left ventricular assist device. J Heart Lung Transplant 2006;25:174-80. [Crossref] [PubMed]
- Xydas S, Rosen RS, Ng C, et al. Mechanical unloading leads to echocardiographic, electrocardiographic, neurohormonal, and histologic recovery. J Heart Lung Transplant 2006;25:7-15. [Crossref] [PubMed]
- Wohlschlaeger J, von Winterfeld M, Milting H, et al. Decreased myocardial chromogranin A expression and colocalization with brain natriuretic peptide during reverse cardiac remodeling after ventricular unloading. J Heart Lung Transplant 2008;27:442-9. [Crossref] [PubMed]
- Hasin T, Kushwaha SS, Lesnick TG, et al. Early trends in N-terminal pro-brain natriuretic peptide values after left ventricular assist device implantation for chronic heart failure. Am J Cardiol 2014;114:1257-63. [Crossref] [PubMed]
- Ahmad T, Wang T, O’Brien EC, et al. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation and renal injury. JACC Heart Fail 2015;3:30-9. [Crossref] [PubMed]
- Sato T, Seguchi O, Iwashima Y, et al. Serum brain natriuretic peptide concentration 60 days after surgery as a predictor of long-term prognosis in patients implanted with a left ventricular assist device. ASAIO J 2015;61:373-8. [Crossref] [PubMed]
- Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357-68. [Crossref] [PubMed]
- Fonarow GC, Peacock WF, Phillips CO, et al. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007;49:1943-50. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis 2003;41:571-9. [Crossref] [PubMed]
- Kato TS, Chokshi A, Singh P, et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail 2011;4:546-53. [Crossref] [PubMed]
- Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol 2008;52:997-1003. [Crossref] [PubMed]
- Papathanasiou M, Pohl J, Jánosi AR, et al. Colonization with multiresistant bacteria: Impact on ventricular assist device patients. Ann Thorac Surg 2018;105:557-63. [Crossref] [PubMed]


