
Immuno-checkpoint inhibitors in metastatic esophago-gastric cancer
Esophageal and gastric cancer show an incidence of 3–4 and 5–6 cases/100,000 people respectively in western countries (1), being the eighth and fifth most common neoplasia worldwide and among the sixth most common cause of cancer related deaths. It is also 3 to 4 times more frequent in men than in women. The most common site of esophageal cancer is the lower third of the organ, involving the esophago-gastric junction (EGJ) with increasing incidence over the last four decades (2); instead the incidence of non-cardia gastric cancers has declined and this trend is likely related to improvements in diet and control of chronic H. pylori infection and concurring increased risk factors such as gastroesophageal reflux disease and obesity. Adenocarcinomas are the most common histological subtype of EGJ cancers (90%) (3).
Due to the peculiar anatomical location, few studies target the single EGJ anatomical site and these patients are typically managed in esophageal and/or gastric cancer treatment trials (4). Indeed, distal esophageal tract adenocarcinomas, EGJ, and gastric cancer show similar survival rates, and similar poor prognosis in case of unresectable, recurrent and metastatic disease (5). Best supportive and palliative cares alone or as simultaneous care are often indispensable for heavily symptomatic patients since chemotherapy feasibility depends upon performance status. Patients who benefit from active cancer treatments receive a first line double regimen with fluoropyrimidines associated to platinum derivatives, such as oxaliplatin or cisplatin, as standard of care (6); moreover, after the recent demonstration of efficacy of the anti-HER2 agent trastuzumab in the treatment of HER2-positive advanced gastric adenocarcinoma, approximately 20% of patients receive the combination of trastuzumab with a chemotherapy doublet (cisplatin and fluoropyrimidine) as treatment of choice (7). A second-line treatment with ramucirumab in combination with paclitaxel chemotherapy showed further significant benefits in terms of progression-free (PFS) and overall (OS) survival, compared with chemotherapy alone, and is actually available for fit patients (8). Nevertheless, prognosis remains poor in presence of metastatic disease and new treatment approaches are desirable.
Consistent with different anatomical site and etiology, four distinct molecular subgroups have been identified, according to The Cancer Genome Atlas (TCGA), in gastro-esophageal cancer (3); these include: (I) Epstein Barr virus (EBV) positive (9%), associated with EBV infection and amplification of potential immune related pathways including over expression of PD-L1 and PD-L2 ligands; (II) microsatellite unstable (MSI) (22%), tumors with high rates of gene hypermethylation and high mutation burden; (III) genomically stable (GS) (20%), tumors with relatively few mutations and presence of CDH-1 and RHO-A mutation; (IV) chromosomal instability (CIN) tumours (50%), genomically unstable tumours with high rates of receptor associated tyrosine kinase pathway gene amplification (HER2, EGFR, MET, FGFR), high rates of p53 mutation, and amplification of VEGFA and cell cycle pathways (9). Notably, EBV-associated tumours and MSI tumours show characteristics that have been associated with high response rates (RRs) to immunotherapy in non-gastric cancer related clinical trials (10). Overall about 40% of gastric and EGJ cancer are PD-L1 positive which make these entities attractive for immunotherapy treatment targeting PD-1 and its ligands.
During these last years, several immune checkpoint inhibitors have consistently improved outcomes for patients with different metastatic tumours, such as melanoma, renal cell carcinoma and non-small-cell lung cancer. On these bases this class of drug have been tested in patients with advanced gastric or EGJ cancer refractory to at least two previous chemotherapy schedules showing encouraging results.
In the ONO-12 (ATTRACTION 2), a randomized phase III study with nivolumab for unresectable advanced or recurrent gastric or EGJ cancer patients refractory to or intolerant to two or more prior chemotherapy regimens, median OS was 5.32 months with nivolumab versus 4.14 months with placebo, and the 12-month OS rate was 26.6% versus 10.9%. In addition, median PFS was 1.61 months for nivolumab versus 1.45 months for placebo. The overall RR was 11.2% with nivolumab versus 0% with placebo, and the median duration of response to nivolumab was 9.53 months (11). Considering the superior survival rates showed in ATTRACTION-2 trial, nivolumab was approved in Japan for the treatment of chemotherapy-refractory gastric and EGJ cancers patients regardless of PD-L1 status. Moreover, in the United States pembrolizumab was approved for the treatment of chemotherapy-refractory PD-L1-positive gastric/EGJ cancer patients based on the KEYNOTE-059 trial (12). In this multicenter, open-label, multicohort trial (KEYNOTE-059/Cohort 1) that enrolled 259 patients with locally advanced or metastatic gastric or EGJ adenocarcinoma was showed durable overall RR. Among the 55% (n=143) of patients whose tumors expressed PD-L1 and either were microsatellite stable or had undetermined MSI or mismatch repair status, the confirmed overall RR was 13.3%; 1.4% had complete responses. Response durations ranged from 2.8 to 19.4 months; 11 patients (58%) had response durations of 6 months or longer, and 5 patients (26%) had response durations of 12 months or longer. Clinical outcomes derived since here from previous trials are reported on Table 1.
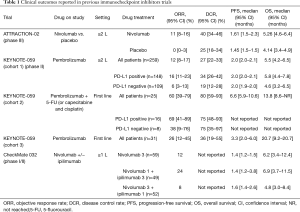
Full table
However, not all patients benefit from single-agent immune checkpoint inhibitor therapy. Actually, most cases of EGJ cancer are CIN, with low immune signature expression and possible low response to immunotherapy.
To address this issue, immunotherapy combinations are increasingly being explored as clinical approach for outcomes improvement even with the evidence of heightened risk of toxicity.
The combinations since here tested have shown complementary mechanisms of immune activity to maximize clinical benefit and minimize immune-related toxicity (13).
In preclinical models dual anti-PD-1 cytotoxic and anti-CTLA-4 demonstrated significant activity (14) and enhanced RRs in patients with metastatic melanoma (15), small cell lung cancer (16), renal cell carcinoma (17) and DNA mismatch repair-deficient (dMMR)/MSI-high (MSI-H) metastatic colorectal cancer (mCRC) (18).
Ipilimumab was the first immune checkpoint therapy used in clinical practice: it improved OS in patients with advanced melanoma, and it was approved by the FDA for the treatment of metastatic melanoma in March 2011. A phase I clinical trial for melanoma patients studied the combination of ipilimumab and nivolumab at escalating doses and response rates (RRs) were compared with each agent as monotherapy. In this trial an increase in immune-related adverse events (irAEs) was reported for the combination therapy (19). The phase II CheckMate 069 study enrolled 142 patients with advanced melanoma, treated with ipilimumab (3 mg/kg) plus nivolumab (1 mg/kg) or ipilimumab alone, showing an overall RR of 61% for combination therapy versus 11% for ipilimumab alone in BRAF wild-type melanoma patients. In the phase III trial CheckMate 067, 945 patients with advanced untreated melanoma were randomized to receive ipilimumab, nivolumab, or concurrent ipilimumab and nivolumab (20). The overall RR for the ipilimumab, nivolumab, and combination arms were 19%, 44%, and 58%, respectively; 3-year OS rates for ipilimumab, nivolumab, and combination therapy were 34%, 52%, and 59%, respectively. However, this study was not built enough to compare nivolumab alone against ipilimumab plus nivolumab. Moreover, it was showed that in advanced melanoma patients significantly longer OS occurred with combination therapy and with nivolumab alone than with ipilimumab alone. In September 2015 the FDA approved the combination immunotherapy for BRAF V600 wild-type unresectable or metastatic melanoma; in April 2018 for intermediate or poor-risk advanced renal cell carcinoma and granted accelerated approval for MSI-H or dMMR mCRC (July 2018).
The CheckMate 032 (21) is a phase I–II trial assessed the safety and efficacy of nivolumab as a single agent or in combination with Ipilimumab in six tumor types—triple-negative breast cancer (TNBC), gastric cancer (GC), pancreatic adenocarcinoma (PC), small cell lung cancer (SCLC), bladder cancer (BC), and ovarian cancer (OC). The study enrolled 160 patients with metastatic esophago-gastric cancer (59 treated with nivolumab 3 mg/kg, 49 with nivolumab 1 mg/kg plus ipilimumab 3 mg/kg, 52 with nivolumab 3 mg/kg plus ipilimumab 1 mg/kg). Seventy-nine percent of patients had received two or more prior therapies. At the data cutoff, objective response rates (ORRs) were 12%, 24%, and 8% in the three groups, respectively.
With a median follow-up of 28, 24, and 22 months across the three groups, 12-month PFS rates were 8%, 17%, and 10%; 12-month OS rates were 39%, 35%, and 24%, respectively. The results with NIVO1 + IPI3 therapy demonstrated an ORR of 24%; however, despite the numerically higher ORR achieved in patients receiving NIVO1 + IPI3 than in those receiving NIVO3, median OS was similar between these groups, mostly related to a higher number of MSI-H and PD-L1-positive tumors patients in the NIVO3 group.
Since here, no biomarker has been shown to be significantly predictive of clinical efficacy from nivolumab plus ipilimumab compared with nivolumab alone.
Tests for PD-L1 expression and mutational burden (MBI) have been studied as predictive indicators of response to immunotherapy in case of PD-L1 positivity and high MBI and they were related with better outcomes (22). Despite the clinical results of nivolumab plus Ipilimumab compared with Nivolumab alone were greatest in PD-L1 negative patients, it was showed how PD-L1 negativity is not a straight predictor of clinical response for combination of immunotherapy compared with a single-agent therapy (15). Accordingly, with the results of the phase III ATTRACTION-2 trial (11) in the CheckMate 032, among EGJ patients, responses were observed regardless of PD-L1 status across the treatment groups, in which PD-L1 expression failed to predict survival; although in CheckMate 032 study the ORR is numerically higher in PD-L1 positive in all subgroups the sample size was too small to be informative. Similarly, the study explores responses in MSI-H and non MSI-H patients: even if ORR seemed numerically higher in the former group however the small sample size does not allow to confirm these findings.
Concerning toxicity, clinical trials conducted on melanoma patients demonstrated higher AEs induced by the combination therapy against ipilimumab or nivolumab alone. For example, in the CheckMate 067 grade 3/4 toxicity rates were 28.3% for ipilimumab, 16.3% for nivolumab, and 55% for combination therapy (15). As expected, the CheckMate 032 demonstrated higher toxicities with combination of inhibitors targeting CTLA-4 and PD-1. Treatment-related grade 3/4 AEs were reported in 17%, 47%, and 27% of patients in the three groups, respectively. In the CheckMate 032 the combination of ipilimumab plus nivolumab is superior to ipilimumab alone in term of ORR but not in term of OS, better if compared with melanoma patients. The clinical matter of whether combination of ipilimumab plus nivolumab is superior to nivolumab alone remains uncertain and need further investigation in phase III studies. The CheckMate 032 also shows highest efficacy for the combination NIVO1 + IPI3, but with a higher incidence of grade 3/4 AEs than observed in NIVO3 group. The question whether the reported excess of toxicity added with the combination immunotherapy has an impact on OS also remain an unsolved issue in this trial and need further investigation. Moreover, remarkable limitations of this study are the absence of a standard-of-care comparator, the small sample size and a design that does not allow a straight comparison across treatment groups.
The interest of recent clinical trials was focused in finding schedules and/or modulating dosing of CTLA-4 inhibitor to minimize irAEs while maintaining similar efficacy (23). Specifically, reducing dose of ipilimumab in combination with anti-PD-1, and\or administrating of ipilimumab less frequently have been investigating strategies. Randomized trials are needed to assess the real efficacy of these combinations with longer-term follow-up (24).
In conclusion, nivolumab and nivolumab plus ipilimumab demonstrated significant antitumor activity associated with durable responses, interesting long-term OS, and manageable toxicity in patients with different tumors. The CheckMate 032 also demonstrated the potential role of combined immune checkpoint modulators in patients with advanced and/or chemotherapy-refractory gastric and EGJ cancers. However, future phase III trials are mandatory and the availability of predictive markers of response in order to define the subpopulation than can achieve the best benefit from a higher toxicity combination therapy is desirable. In clinical practice an important issue for the choice of immunotherapy in any setting is the patient capability to handle the irAEs according to his specific clinical condition. Patients who do not have supportive caregivers or with poor performance status could not be the best candidates for combination immunotherapy, considering the potential toxicity management that necessitate adherence to immunosuppressive treatment regimens in case of significant immunotoxicity. For these reasons, single agent anti-PD-1 therapy remains an appropriate choice for fragile patients and it should be considered the standard control arm for future randomized clinical trials.
Concerning esophagogastric cancer the role of immunotherapy in different setting of the disease need to be clarified. Phase III studies evaluating nivolumab or nivolumab plus ipilimumab in earlier lines (neoadjuvant and adjuvant) of therapy are ongoing and needed. Further studies on associating immunotherapy with standard chemotherapy are also needed.
The clinical approach for future studies should be directed to evaluate when (earlier versus later lines therapy) and how (alone or in combination) to include nivolumab and nivolumab plus ipilimumab into clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [Crossref] [PubMed]
- Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001;92:549-55. [Crossref] [PubMed]
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010;8:437-47. [Crossref] [PubMed]
- Huang PM, Chen CN. Therapeutic strategies for esophagogastric junction cancer. Formosan Journal of Surgery 2015;48:185-97. [Crossref]
- Whitson BA, Groth SS, Li Z, et al. Survival of patients with distal esophageal and gastric cardia tumors: a population-based analysis of gastroesophageal junction carcinomas. J Thorac Cardiovasc Surg 2010;139:43-8. [Crossref] [PubMed]
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010.CD004064. Update in: Chemotherapy for advanced gastric cancer. [Cochrane Database Syst Rev 2017]. [PubMed]
- Bittoni A, Del Prete M, Scartozzi M, et al. Three drugs vs two drugs first-line chemotherapy regimen in advanced gastric cancer patients: a retrospective analysis. Springerplus 2015;4:743. [Crossref] [PubMed]
- Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol 2016;22:2403-14. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy 2016;8:821-37. [Crossref] [PubMed]
- Selby M, Englehardt J, Lu LS, et al. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J Clin Oncol 2013;31:abstr 3061.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The CheckMate 016 Study. J Clin Oncol 2017;35:3851-8. [Crossref] [PubMed]
- Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 2018;36:773-9. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017;377:1345-56. [Crossref] [PubMed]
- Janjigian Y, Bendell JC, Calvo E, et al. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (NIVO) alone or with ipilimumab (IPI) in advanced and metastatic (A/M) gastric cancer (GC). J Clin Oncol 2016;34:abstr 4010.
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542-51. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Postow M. Reduced-dose ipilimumab with standard-dose pembrolizumab: is less more? Lancet Oncol 2017;18:1144-5. [Crossref] [PubMed]


