
Prediction of response to endobronchial coiling based on morphologic emphysema characterization of the lung lobe to be treated and the ipsilateral non-treated lobe as well as on functional computed tomography-data: correlation with clinical and pulmonary function
Introduction
Endoscopic lung volume reduction (ELVR) techniques for the treatment of patients with advanced chronic obstructive pulmonary disease (COPD) have increasingly gained acceptance and replaced surgical treatments due to less associated morbidity and mortality (1,2). Of the different endoscopic treatment options, endobronchial valve or coil implantation has gained particular attention with growing data in support of their effectiveness (3-5). However, the target populations and the underlying pathomechanisms of the two techniques differ significantly.
Bronchial valve implantation is expected to reduce hyperinflation of destroyed emphysematous lung parenchyma by up to 80% in case of a complete interlobar fissure and heterogeneous emphysema phenotype (6,7). The integrity of the interlobar fissure is considered equal to invasive measurements of collateral ventilation, which annihilates the beneficial effect of one-way valves if present (7-10).
Endobronchial coiling represents an alternative ELVR technique, which acts by torquing and retracting the bronchi and nearby lung tissue, which stabilizes the bronchial tree and the adjacent lung parenchyma, thereby restoring to some extent the elastic recoil and improving respiratory function (11). Therefore, endobronchial coiling is independent of collateral ventilation and may be indicated for patients who do not qualify for valve implantation (11). Bronchial coiling has been used predominantly to treat patients with heterogeneous emphysema phenotypes (12,13), whereas later endobronchial coiling has also shown a beneficial effect in patients with homogeneous emphysema distribution (14,15).
Chest computed tomography (CT) has emerged as a major diagnostic tool for selecting patients for ELVR techniques due to its ability to characterize emphysema and pulmonary fissures (6,16). Visual assessment and now more widely available emphysema quantification are helpful for the detection and classification of homogeneous and heterogeneous phenotypes, including defining the completeness of the interlobar fissure and target selection of the lobe with the highest degree of destruction.
The purpose of our study was to test if the emphysema type of the targeted lobe, ipsilateral non-targeted lobe, and lobes of the contralateral lung impact outcome of ELVR treatment, and to document lobar volume changes in treated and non-treated lung lobes.
Methods
Patient characteristics
The ethics board of the Medical Faculty and the University Hospital of the Eberhard-Karls University approved this retrospective data evaluation study and waived the informed consent requirement (No. 198/2016BO1).
A search of our patient information system derived 30 patients who underwent endobronchial coiling therapy for LVR at our institution between December 2011 and March 2016.
Inclusion and exclusion criteria were similar to previously reported studies in the literature (17). Main inclusion criteria were the presence of severe chronic obstructive lung disease (COLD), non-smoker (>6 months), RV >200% of predicted and absence of acute pulmonary infection. We included only patients who underwent CT examination at end-inspiratory phase before and after therapy with at least one follow-up examination. Main exclusion criteria included a change in FEV1 >15% post-bronchodilator, single-breath Hb (hemoglobin) corrected diffusion capacity (DLCOC-SB) <20% predicted, CT examination with evidence of diffuse emphysema, previous lung surgery, and relevant lung diseases (e.g., bullae, bronchiectasis, lung cancer) which might have adversely affected the outcome of the study.
Twenty-eight of the 30 (93.3%) patients had stage IV COLD, and 2 (6.7%) patients had stage III COLD. Twenty-one of the patients had smoker anamnesis (70%; mean, 36.5 pack years ±13.5).
Eleven patients (36.7%) needed permanent oxygen therapy with a mean consumption of 2.3 liter/min. Twelve patients (40.0%) had nocturnal ventilation (NIV). An average of 15.1 coils was implanted per patient [standard deviation (SD) 5.3; range, 4–22 coils]. The distribution of the coils is shown in Table 1. Figures 1,2 show examples for each side.

Full table
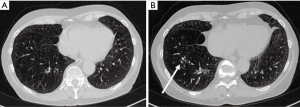
Baseline chest CT examinations were performed at a mean of 54.63 days (SD ±49.69 days; range, 0–105 days) before endobronchial coiling. The indication for high-resolution chest CT was made by a board-certified pulmonologist in all cases for the planning of the intervention and evaluation of results after implantation of the coils. Follow-up CT examinations were performed 121.67±147.19 days after intervention and served for evaluation of results in combination with clinical and pulmonary functional tests. A total of 97 chest CT examinations were performed including 30 baseline and 67 follow-up examinations. The mean number of CT examinations was 3±1.4 (range, 1–7 examinations per patient). All chest CT examinations were retrospectively evaluated and excluded if signs of infection or non-infectious complications like edema or hemorrhages were present. One patient had prior LVR surgery of one lung and was excluded from the final analysis. Four patients had prior contralateral endobronchial therapy for LVR with valves; however, at the time of measurement, no further valves were implanted.
High-resolution CT-technique
CT examinations were performed using a 128 row multidetector CT scanner (Somatom Definition AS Plus, Siemens Medical Systems, Erlangen, Germany), a 512×512 reconstruction matrix, a photon energy of 120 kV, a tube current of 100–150 effective mAs, and a tube rotation time of 0.5 ms. The field-of-view was adjusted for each patient to include the entirety of the chest wall and both lungs.
No IV contrast material was applied. In all patients, a spiral acquisition was obtained from the apex to the base of the lungs at end-inspiratory phase. Examinations were performed with patients in the supine position. For visual assessment, two separate CT image sets with 3 mm thick slices were created, using soft (B31f filter) and sharp (B70f filter) reconstruction algorithms. For CT densitometry and lung volume calculation, an additional CT image set with 0.6 mm thick slices was created using soft tissue kernel (B31f filter) reconstruction algorithms.
Qualitative CT imaging analysis
All CT images were viewed at standard mediastinal (level, 35 HU; width, 450 HU) and lung (level, −700 HU; width, 1,500 HU) window settings.
Quantitative CT image analysis
We used “Syngo CT Pulmo 3D” software (SyngoVia, Siemens Healthcare, Germany) to evaluate the airway system using image data sets with 0.6 mm thick slices. The imaging analysis process and the underlying software steps have been described elsewhere (5,6). The lung volumes of each patient were measured separately for each lung lobe. The lung and the different lobes were first automatically detected by the software and additionally manually corrected if the interlobar fissures were inaccurately delineated. We additionally confirmed the results of computer-based segmentation in the coronal and transversal planes.
Two radiologists (BLINDED and BLINDED) with 24 and 4 years of experience in interpreting chest CT examinations performed the analysis independently from each other. Both investigators were blinded to the original interpretation and the clinical situation of each patient. The emphysema phenotype was classified in each lobe using Syngo CT Pulmo 3D software (Siemens Healthcare), utilizing whole lung color-coded maps displaying the number and distribution of emphysema equivalent (density <−950 HU) lung parenchymal areas (clusters). Based on this “cluster” analysis, we classified the emphysema in each lung lobe into homogenous (almost no parenchymal area exhibiting attenuation values >−950 HU left) and heterogeneous [a mixture of parenchymal areas (patchy pattern) showing attenuation values >−950 and <−950 HU] types.
Clinical tests
Pulmonary function tests (PFT) were performed in all patients before and after endobronchial coiling according to the European Respiratory Society guidelines. PFT was performed using a Masterscreen Body plethysmograph (CareFusion GmbH, Hoechberg, Germany) in the pre-interventional setting and periodically (every 3–6 months) thereafter. Following PFT, total lung capacity (TLC), residual volume (RV), forced expiratory volume in 1 s (FEV1), and single-breath diffusion capacity for carbon monoxide (DLCOcsb) were quantified. Additionally, every patient underwent a blood gas analysis, a 6-minute walking test (6MWT), and COPD assessment test (CAT) questionnaire. Based on changes in the 6MWT that was performed 6 months after the LVR therapy, patients were classified into two groups of responders and non-responders.
Statistics
Statistical analysis was performed using dedicated software (IBM SPSS22.0, SPSS, Armonk, USA). All results are expressed as mean average with standard deviation. The Kolmogorov-Smirnov test was used for the normality test including Lilliefors significance correction. Paired t-test was used for significance testing concerning 6MWT and CAT. Wilcoxon signed rank test was used for significance testing blood gas values and PFT parameters before and after the intervention. For comparison of lung volumes and emphysema type before and after treatment Wilcoxon signed rank test was used. Paired t-test was used for significance testing concerning mean lung density (MLD), high-attenuation values (HAV), and low-attenuation values (LAV). For comparing responders and non-responders, repeated measurements with Bonferroni correction were used. For each comparison, the P value for testing between the groups and time points is given. All tests were corrected for multiple measurements. Chi-square test and McNemar’s test were used for analyzing emphysema distribution. Kruskal-Wallis test was used for testing difference in responders after treatment in upper and lower lobes. A value of P<0.025 was considered significant.
Results
Response classification to endobronchial coiling based on 6MWT
Based on changes in the 6MWT that was performed 6 months after LVR therapy, patients were classified into responders and non-responders. Accordingly, 19 were classified into responders (63.3%) with a change from 281.05±88.31 meters to 335.26±93.47 (P=0.001) meters, whereas 11 were classified as non-responders (36.7%) yielding a mean walking distance of 308.18±92.82 meters and 255.45±99.13 meters after intervention (P=0.001).
In 16/30 patients, the interlobar fissure was moderately or severely incomplete on both sides, whereas 8/16 patients with incomplete fissure were responders and 8/16 patients were non-responders.
Other clinical parameters (CAT, blood gas analysis, PFT)
Clinical parameters are shown in Table 2. In responders, the CAT test decreased from 23.23±7.31 to 20.73±6.10 points (P=0.038), whereas in non-responders there was a decrease from 24.44±7.29 to 24.36±4.29 points (P=0.092). In responders, there was a significant decrease in pCO2 from 42.94±10.87 to 40.31±4.91 (P=0.001), whereas in non-responders there was a significant increase in pCO2 from 44.27±10.70 to 47.45±9.63 (P=0.003). Table 3 shows the results of PFT in responders and non-responders.

Full table
Full table
Lung volumes and CT quantification
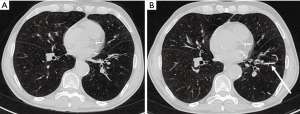
In responders, there was a statistically significant volume reduction of the treated lobe from 1,627.68±531.75 mL before to 1,519.21±458.47 mL after therapy (P=0.009), whereas in non-responders, there was an increase of volume from 1,424.18±335.38 mL before, to 1,428.36±317.15 mL after therapy (P=0.722). Ipsilateral non-targeted lobes of both responders and non-responders showed a strong, but non-significant tendency of increasing volumes after therapy from 1,073.18±607.88 to 1,106.56±652.09 mL (P=0.137) and 1,015.76±516.70 to 1,034.52±528.10 mL (P=0.455), respectively. The LAV decreased in the treated lobe in responders and increased in non-responders, whereas HAV slightly decreased from 30.98±8.93 to 27.41±7.35 HU and increased in non-responders from 24.05±5.63 to 25.46±5.06 HU (Table 4). Figures 3,4 show representative cases.

Full table
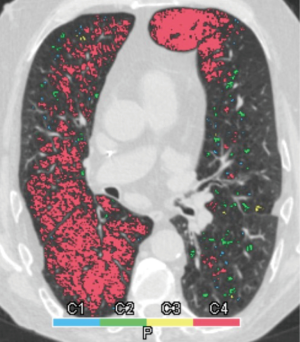
Emphysema phenotype
The distribution of emphysema types between the treated, ipsilateral untreated, and contralateral lobes are shown in Table 5. There was no significant difference between responders and non-responders (P=0.825). Overall, the distribution of emphysema showed a significant difference between the lobes (P=0.005) before therapy in both ipsilateral and contralateral non-treated lung lobes, but not in treated lobes. In non-responders, there were no significant differences between emphysema phenotypes before (P=0.280) and after therapy (P=0.378). In responders, the emphysema phenotype of treated (P=0.999), ipsilateral non-treated (P=0.687), and contralateral lobes (P=0.250) did not change significantly after therapy. Similarly in non-responders, the emphysema phenotype of treated (P=0.250), ipsilateral non-treated (P=0.999), and contralateral lobes (P=0.999) did not change significantly either. There were no significant differences between treated upper and lower lobes (P=0.310) in responders.
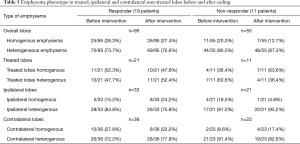
Full table
Receiver operating characteristics (ROC)
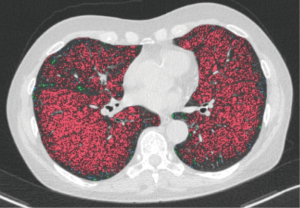
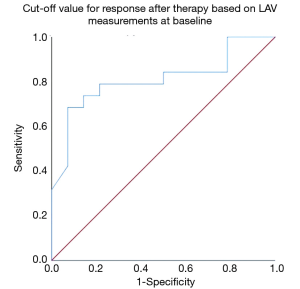
The cuff-off value for a response after therapy based on LAV measurements at baseline was significant for a LAV of 23.47 or lower in inspiration (sensitivity/1-specificity/AUC/P value 78.59%/50.0%/0.792/0.007) (Figure 5). There were no significant cuf-off values for MLD, HAV, and PFT.
Discussion
The selection of the most appropriate lobe for lung volume reduction (LVR) with endobronchial coiling is important to optimize outcomes in patients with severe COLD. For this purpose, different morphologic and functional methods have been proposed, which are mostly based on the differentiation of homogeneous and heterogeneous emphysema phenotypes (16,18,19). While this differentiation can be performed visually on high-resolution CT images of the chest, quantification of lung parenchymal density may be more accurate, and calculation of the distribution of emphysema equivalent areas has become practicable based on the so-called “cluster analysis” (20). In order to understand which impact the interplay of different lobar phenotypes could potentially have in a patient, we performed lobar-based phenotype analysis of the targeted lobe, and also of the nontargeted ipsilateral and contralateral lung lobes.
We found that the emphysema phenotype in the targeted lobe has no association with the response to endobronchial coiling. Our data support more recent reports, which similarly found that the emphysema phenotype may not influence the treatment outcome (21). Interestingly, the emphysema phenotype in the ipsilateral, untreated lung lobes also did not influence the patient outcome; however, there was a predominance of heterogeneous emphysema phenotypes in our cohort. We found no relevant difference in terms of response between the groups with either homogeneous emphysema of treated and non-treated ipsilateral lobes or heterogeneous emphysema of treated and non-treated ipsilateral lobes. The same was true also for the contralateral lung lobes. Hence, the choice of the most proper lung lobe to be treated by endobronchial coiling seems indeed not to be influenced by the emphysema phenotype, which is in contrast to valve-based LVR (16). Accordingly, the pre-interventional work-up of patients undergoing endobronchial coiling may be simplified compared to other LVR procedures. Nonetheless, for evaluation of response and treatment monitoring, CT densitometry and volumetry of lung parenchyma proved very useful (22). Notably, classification of response in our series was based on results of 6MWT, which were also in concordance with those of the CAT test and blood gas analysis. However, the PFT parameters which are used for routine patient evaluation did not change significantly either in the responder or non-responder group, indicating a major limitation for routine response evaluation in this clinical setting. Our non-responder group had worse PFT at baseline, which is similar to previous studies (5,14). However, the difference in the absolute values was more extensive in our group, which suggests that the effectiveness of coiling may be limited in the setting of poor baseline PFT parameters.
To the best of our knowledge, this is the first report considering not only the impact of the emphysema phenotype of the treated lobe but also of the ipsilateral and contralateral lobes, and thus provides an opportunity to understand the potential mechanical effect on the post-interventional respiratory function. The strategy of pre-interventional lobe selection for endobronchial LVR has been addressed previously (2,16). Generally, the lobes with the greatest degree of destruction have been chosen for endobronchial treatment. However, at least for valve placement, the consideration of the emphysema phenotype is still an issue of debate. More recent data have emphasized the role of lobar deflation in expiration, showing that morphological criteria based exclusively on inspiratory CT densitometry only partially reflect the deflation of particular lung lobes, and may be of limited value as a sole predictor for target lobe selection in LVR. Contrary to endobronchial valve implantation, which aims at volume reduction of the most affected (destroyed) lung lobe by precluding inflation through a one-way bronchial valve, coiling leads to mechanical volume reduction through the distribution of increased radial tension throughout the airway network, while tethering opens small airways to prevent collapse (23). Coiling also causes a significant decrease in the cross-sectional area of treated segment bronchi in inspiration and a slight increase in expiration accompanied by a volume reduction and stabilizes the bronchial tree (5). Our data support this pathomechanistic interpretation of the role of endobronchial coiling in that there is no relevant change in volume and attenuation of the other lung lobes, but only of the treated lobe after endobronchial coiling. Only in responders did we find a slight volume increase of the ipsilateral lobe and a mild decrease in density. However, improvement in respiratory function seemed mainly secondary to the decrease of hyperinflation of treated lung lobes.
In our cohort, 8/30 patients (26.6%) showed lower lobe-dominant emphysema. In 4 cases, these patients were responders, and in 4 cases the patients were non-responders. According to the current literature (24), our group showed an upper lobe dominant emphysema pattern; however, without consequence for the outcome.
Our study has limitations. First, the number of treated lobes is relatively low for comprehensive statistical analysis. Second, we had a predominance of heterogeneous emphysema phenotype in the ipsilateral and contralateral non-treated lobes, which may have also influenced comprehensive statistical analysis.
In conclusion, we found that the phenotype of lung emphysema in the targeted and non-targeted ipsilateral lobes had no significant impact on the outcome of endobronchial coiling for LVR and also did not change significantly after treatment, whereas statistically significant changes in lobar volume were found solely in the treated lobe in responders.
Acknowledgements
The authors thank Claudia Kroll (technical assistant) for the sustained support in conducting this study.
Footnote
Conflicts of Interest: J Fritz received institutional research funds and speaker’s honorarium from Siemens Healthcare USA and is a scientific advisor of Siemens Healthcare USA. The other authors have no conflicts of interest to declare.
Ethical Statement: The ethics board of the Medical Faculty and the University Hospital of the Eberhard-Karls University approved this retrospective data evaluation study and waived the informed consent requirement (No. 198/2016BO1).
References
- Eberhardt R, Gompelmann D, Herth FJ, et al. Endoscopic bronchial valve treatment: Patient selection and special considerations. Int J Chron Obstruct Pulmon Dis 2015;10:2147-57. [PubMed]
- Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial valves for endoscopic lung volume reduction: Best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration 2017;93:138-50. [Crossref] [PubMed]
- Eberhardt R. Endobronchial valve placement in emphysema: When is it lung volume reduction? Respirology 2018;23:242-3. [Crossref] [PubMed]
- van Agteren JE, Hnin K, Grosser D, et al. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;2:CD012158. [PubMed]
- Kloth C, Thaiss WM, Hetzel J, et al. Impact of endobronchial coiling on segmental bronchial lumen in treated and untreated lung lobes: Correlation with changes in lung volume, clinical and pulmonary function tests. Eur Radiol 2016;26:2176-83. [Crossref] [PubMed]
- Grosse U, Hetzel J, Gündel L, et al. Impact of endobronchial coiling for lung volume reduction on pulmonary volume and attenuation: preinterventional and postinterventional computed tomography-quantification using separate lobe measurements. J Comput Assist Tomogr 2014;38:779-85. [Crossref] [PubMed]
- Hopkinson NS. Endobronchial Valves as a Treatment for Emphysema. Moving out of the Shadow of Lung Volume Reduction Surgery. Am J Respir Crit Care Med 2016;194:1039-40. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Reymond E, Jankowski A, Pison C, et al. Prediction of lobar collateral ventilation in 25 patients with severe emphysema by fissure analysis with CT. AJR Am J Roentgenol 2013;201:W571-5. [Crossref] [PubMed]
- Koster TD, Slebos DJ. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis 2016;11:765-73. [Crossref] [PubMed]
- Klooster K, Ten Hacken NH, Franz I, et al. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration 2014;88:116-25. [Crossref] [PubMed]
- Kontogianni K, Gerovasili V, Gompelmann D, et al. Effectiveness of endobronchial coil treatment for lung volume reduction in patients with severe heterogeneous emphysema and bilateral incomplete fissures: a six-month follow-up. Respiration 2014;88:52-60. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Herth FJ. Endoscopic lung volume reduction. A European perspective. Ann Am Thorac Soc 2013;10:657-66. [Crossref] [PubMed]
- Herth FJ, Eberhard R, Gompelmann D, et al. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 2010;4:225-31. [Crossref] [PubMed]
- Klooster K, Ten Hacken NH, Slebos DJ. The lung volume reduction coil for the treatment of emphysema: a new therapy in development. Expert Rev Med Devices 2014;11:481-9. [Crossref] [PubMed]
- Hetzel J, Boeckeler M, Horger M, et al. A new functional method to choose the target lobe for lung volume reduction in emphysema - comparison with the conventional densitometric method. Int J Chron Obstruct Pulmon Dis 2017;12:2621-8. [Crossref] [PubMed]
- Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142:574-82. [Crossref] [PubMed]
- Kim EY, Seo JB, Lee HJ, et al. Detailed analysis of the density change on chest CT of COPD using non-rigid registration of inspiration/expiration CT scans. Eur Radiol 2015;25:541-9. [Crossref] [PubMed]
- Washko GR, Criner GJ, Mohsenifar Z, et al. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD 2008;5:177-86. [Crossref] [PubMed]
- Kloth C, Thaiss WM, Ditt H, et al. Segmental bronchi collapsibility: computed tomography-based quantification in patients with chronic obstructive pulmonary disease and correlation with emphysema phenotype, corresponding lung volume changes and clinical parameters. J Thorac Dis 2016;8:3521-9. [Crossref] [PubMed]
- Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233-40. [Crossref] [PubMed]
- Kloth C, Maximilian Thaiss W, Preibsch H, et al. Quantitative chest CT analysis in patients with systemic sclerosis before and after autologous stem cell transplantation: comparison of results with those of pulmonary function tests and clinical tests. Rheumatology (Oxford) 2016;55:1763-70. [Crossref] [PubMed]
- Zoumot Z, Kemp SV, Singh S, et al. Endobronchial coils for severe emphysema are effective up to 12 months following treatment: medium term and cross-over results from a randomised controlled trial. PLoS One. 2015;10:e0122656. [Crossref] [PubMed]
- Theilig D, Doellinger F, Poellinger A, et al. Comparison of distinctive models for calculating an interlobar emphysema heterogeneity index in patients prior to endoscopic lung volume reduction. Int J Chron Obstruct Pulmon Dis 2017;12:1631-40. [Crossref] [PubMed]


