
Prophylactic cranial irradiation in non-small cell lung cancer: the debate is open
Non-small cell lung cancer (NSCLC) is diagnosed in about 30% of patients as locally advanced disease (stage III) which is defined as a tumour that exceeds the structures of the lung itself, but without clinical evidence of distant spreading. These patients form a highly heterogeneous group with controversial treatment based on the combination of surgery, chemotherapy and radiotherapy (1).
Brain metastases (BMs) develop in approximately 20–40% of NSCLC patients at some point during their disease course (2). Their incidence is increasing because of the aging population, improvements in the systemic therapies, and advances in imaging modalities, such as magnetic resonance imaging (MRI), to detect small metastases at follow-up screening examinations (2). The prognosis for patients with BMs is detrimental and depends on age, performance status, number of BMs, systemic disease control, and presence of neurological symptoms. Generally, the median overall survival (OS) is about 7 months (3). BMs are also associated with a negative impact on quality of life (QoL). Thus, these are becoming an unmet need for the management of NSCLC patients.
Prophylactic cranial irradiation (PCI) decreases the incidence of BMs in localized small cell lung cancer (SCLC) and can lead to improved long-term OS (4). This approach has also been investigated in the treatment of localized NSCLC (5).
Recently the phase III study of the NVALT/DLCRG groups investigating whether PCI reduces the incidence of symptomatic BMs in patients with stage III NSCLC treated with curative intention was published. The primary endpoint was defined as the occurring of one or a combination of key symptoms suggesting BMs (signs of increased intracranial pressure, headache, nausea and vomiting, cognitive or affective disturbances, seizures, and focal neurologic symptoms) together with MRI or computed tomography (CT) scan confirming the existence of BMs, at 24 months from concurrent/sequential chemo-radiotherapy with or without surgery. A total of 175 patients were randomized to PCI or observation and with a median follow-up of 48.5 months, PCI showed to reduce symptomatic BMs incidence at 2 years from 27.2% in the control group to 7% in the PCI group (P<0.001). It also delayed the time to develop symptomatic BMs with a hazard ratio (HR) of 0.23 [95% confidence interval (CI): 0.09–0.56; P=0.0012]. However, OS was not different between the two arms and neurologic adverse events scored by the physician resulted increased in the PCI group. In fact, median OS was 24.2 months in the PCI arm and 21.9 months in the control arm (HR 0.9, 95% CI: 0.62–1.29; P=0.56). Median progression-free survival was 12.3 and 11.5 months, respectively (HR 0.79, 95% CI: 0.56–1.11; P=0.17). Grade 1 and 2 memory impairment was 30% in the PCI arm and 8% in the control arm and cognitive disturbance was 18.5% and 3.5%, respectively. QoL, measured by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30, and EuroQol 5D measurement, resulted decreased only 3 months post-PCI and was similar to the observation arm thereafter (6).
This trial confirmed the highly significant results in favor of PCI in terms of BMs incidence reduction already reported by previous randomized studies addressed to stage III NSCLC (7-9) (Table 1) [13% reduction, relative risk (RR) 0.33, 95% CI: 0.22–0.45] without a corresponding OS benefit (5). This lack of OS benefit may be related to the absence of systemic disease control which can lead to death for extracranial failures or to the increased central nervous system (CNS) control in patients not undergoing PCI related to the use of stereotactic brain radiotherapy (SRT) in case of asymptomatic BMs (5).
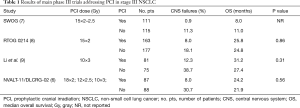
Full table
Based on these results, can we recommend PCI in stage III NSCLC patients? Why can the impressive symptomatic BMs reduction not be enough to deliver PCI in these patients?
Firstly, there is a lack of data on the role of MRI surveillance associated with brain SRT. In fact, MRI can detect a very high rate of asymptomatic metastases (up to 89%) and SRT showed to be safe and effective also in patients with more than 4 metastases without cognitive impairment (10).
Secondly, PCI dose to be delivered in NSCLC is still to be established. Conversely, a randomized trial showed that a standard PCI dose of 25 Gy was not inferior and associated with lower related toxicity compared to a higher dose (36 Gy) in patients with limited-stage (LS) SCLC (11).
Thirdly, new data from the phase III PACIFIC trial of durvalumab after chemoradiotherapy in patients with stage III NSCLC showed that the drug increases the odds of surviving for 24 months (12).
If the survival prolongation can result in increased risk of BMs and therefore in a larger benefit from PCI, it may also be associated with a longer time to develop late radiotherapy-related cognitive effects. For this reason, a baseline evaluation of neurocognitive patients’ abilities should be performed before undergoing PCI. In fact, in SCLC patients yet to undergo PCI, the evaluation revealed that 47% of them had already evidence of impaired cognitive function (13). Thus, strategies to preserve cognition in patients with BMs undergoing PCI should be investigated. In this context, a possible strategy could be to deliver whole brain radiotherapy (WBRT) but reducing radiation exposure to the hippocampus. This area, accounting for only 5% of BMs in SCLC and less than 3% in NSCLC (14), has been supposed to harbour proliferating neuronal progenitor cells responsible for radiation-induced neurocognitive decline. Preliminary data from a multi-institutional single-arm phase II RTOG 0933 trial demonstrated superior cognitive preservation with hippocampal avoidance WBRT (HAWBRT) (15). Currently the phase II/III NRG CC003 (NCT02635009) is investigating the role of HAWBRT in limiting cognitive impairment without increasing intracranial relapse rate in LS-SCLC. A further strategy to limit the neurocognitive effect of PCI relies in the use of neuroprotective drugs to preserve cognitive function. Memantine, an antagonist of the N-methyl-D-aspartate (NMDA) receptor involved in learning and memory, showed in a randomized trial (RTOG 0614) to delay time to cognitive decline and reduced the rate of decline in memory, executive function and processing speed when delivered concurrently with WBRT and for the subsequent 6 months (16). Donepezil, a reversible acetylcholine esterase inhibitor, even if failed to improve cognitive scores, obtained memory improvement when given in association with WBRT (17). Furthermore lithium, a drug widely employed for treating bipolar disorder, is currently being evaluated in preventing or reducing memory problems in patients treated with PCI.
Finally, in the era of precision medicine, the intracranial efficacy of molecular-targeted therapy and immunotherapy needs to be addressed. Unfortunately, most of the available data come from metastatic NSCLC patients. While targeted inhibitors have clear data concerning both the treatment and the prevention of BMs, the results reported in this subgroup of patients with immune checkpoints inhibitors are still preliminary but seem promising (18). Moreover, new research could identify criteria to predict the spatial distribution of BMs according to the primary tumour (19). This information could be of particular interest to select areas at higher/lower risk for BMs. In addition, considering that the cerebral function is particularly complex and different from individual to individual, imaging able to identify the functional areas of the brain could be a useful tool to optimize the therapeutic approach. Important advances have been made in brain imaging, especially with functional mapping and fibre tracking with the use of diffusion tensor imaging. Integration of these technologies to improve planning of radiotherapy could be a great opportunity to minimize sequelae. In particular, thanks to the highly conformed modern radiotherapy techniques today it is possible to modulate the dose distribution so as to reduce the exposure of the most involved areas in the development of neurocognitive damages (20). Probably, in the next future newer data will be available in these settings to define new therapeutic strategies for BMs prevention and management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep 2012;14:48-54. [Crossref] [PubMed]
- Lee DH, Han JY, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: result of a randomized pilot study. Cancer 2008;113:143-9. [Crossref] [PubMed]
- Soffietti R, Abacioglu U, Baumert B, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 2017;19:162-74. [Crossref] [PubMed]
- Witlox WJA, Ramaekers BLT, Zindler JD, et al. The prevention of brain metastases in non-small cell lung cancer by prophylactic cranial irradiation. Front Oncol 2018;8:241. [Crossref] [PubMed]
- De Ruysscher D, Dingemans AC, Praag J, et al. Prophylactic cranial irradiation versus observation in radically treated stage III non-small-cell lung cancer: A randomized phase III NVALT-11/DLCRG-02 study. J Clin Oncol 2018;36:2366-77. [Crossref] [PubMed]
- Miller TP, Crowley JJ, Mira J, et al. A randomized trial of chemotherapy and radiotherapy for stage III non-small cell lung cancer. Cancer Ther 1998;1:230-36.
- Gore EM, Bae K, Wong SJ, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of Radiation Therapy Oncology Group study RTOG 0214. J Clin Oncol 2011;29:272-8. [Crossref] [PubMed]
- Li N, Zeng ZF, Wang SY, et al. Randomized phase III trial of prophylactic cranial irradiation versus observation in patients with fully resected stage IIIA-N2 nonsmall-cell lung cancer and high risk of cerebral metastases after adjuvant chemotherapy. Ann Oncol 2015;26:504-9. [Crossref] [PubMed]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [Crossref] [PubMed]
- Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467-74. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Grosshans DR, Meyers CA, Allen PK, et al. Neurocognitive function in patients with small cell lung cancer: effect of prophylactic cranial irradiation. Cancer 2008;112:589-95. [Crossref] [PubMed]
- Harth S, Abo-Madyan Y, Zheng L, et al. Estimation of intracranial failure risk following hippocampal-sparing whole brain radiotherapy. Radiother Oncol 2013;109:152-8. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi institutional trial J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole brain radiotherapy: a randomized, double-blind, placebo controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]
- Rapp SR, Case LD, Peiffer A, et al. Donezepil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol 2015;33:1653-9. [Crossref] [PubMed]
- Liao BC, Lin CC, Yang JC. Treating brain metastases in non-small cell lung cancer patients: what have we learnt from pharmaceutical recent clinical trials? Expert Opin Pharmacother 2018;19:851-64. [Crossref] [PubMed]
- Quattrocchi CC, Errante Y, Mallio CA, et al. Inverse spatial distribution of brain metastases and white matter hyperintensities in advanced lung and non-lung cancer patients J Neurooncol 2014;120:321-30. [Crossref] [PubMed]
- Ajithkumar T, Price S, Horan G, et al. Prevention of radiotherapy-induced neurocognitive dysfunction in survivors of paediatric brain tumours: the potential role of modern imaging and radiotherapy techniques. Lancet Oncol 2017;18:e91-100. [Crossref] [PubMed]


