
Can treatment outcomes of latent TB infection and TB in silicosis be improved?
Occupational exposure to silica dust (respirable crystalline silica) leading to the development of silicosis still occurs in many parts of the world, including countries such as South Africa, Turkey, China and Sri Lanka. Immune dysfunction (or dysregulation), through involvement of host macrophages and lymphocytes alongside their elaborated cytokines, resulting in exaggerated humoral response and suppression of cell-mediated immunity, is believed to be important mechanisms of pathogenesis in this form of pneumoconiosis (1). Inflammation and fibrosis are well known pathological changes in the lungs of patients inflicted with silicosis, resulting in compromised respiratory function. Furthermore, these unfortunate patients are also at an increased risk of developing active pulmonary tuberculosis (TB), largely through endogenous reactivation of latent infection due to Mycobacterium tuberculosis (2), which is prevalent in many countries and geographical areas worldwide. Thus, TB in silicotic subjects poses an important challenge to global lung health. Preventive therapy for TB in patients with silicosis is beneficial. In a double-blind placebo-controlled clinical trial of 3 anti-TB regimens, namely isoniazid monotherapy, rifampicin monotherapy and combined therapy with isoniazid and rifampicin, the estimated proportions of patients with active pulmonary TB were about half of those who received placebo, 5% versus 9% at 2 years, 8% versus 15% at 3 years, 10% versus 20% at 4 years and 13% versus 27% at 5 years of study (3). However such results still appear somewhat inferior to the generally recognised efficacy of treatment of latent TB infection of about 60–90% (2). A short-course (3-month) chemoprophylaxis regimen comprising rifampicin, isoniazid and pyrazinamide in combination also has not been shown to effectively prevent the development of active TB in miners with silicosis, as revealed by the results of a randomised double-blind placebo controlled trial (4), although the study findings might have been confounded by a high risk of TB reinfection among gold mine workers that could have offset treatment protective effect, as also alluded to in another isoniazid preventive therapy trial (5). Several years ago, the use of 12 doses of weekly rifapentine and isoniazid has been shown to be as effective as 9 months of daily isoniazid for treating latent TB infection in a clinical trial (6). However, as the study did not clearly provide the number of included subjects with silicosis, it is not possible to firmly extrapolate that this short-course regimen comprising once-weekly administration of rifapentine and isoniazid works equally well in the silicotic subjects. Apparently, dedicated studies regarding “new” TB preventive therapy regimen(s) for this specific population are required to address the question better.
The treatment of TB disease in silicotic patients is also problematic. In a controlled clinical trial regarding silico-TB, patients were randomised to receive 6 months or 8 months of three-times-weekly treatment with streptomycin, isoniazid, rifampicin and pyrazinamide in combination (7). Overall, only 80% of patients achieved sputum culture conversion to negativity after 2 months. 22% of patients who received 6 months of treatment had relapse of TB during the total 3-year assessment whereas 7% of those with 8-month treatment did so. Toxicities induced by the 4-drug regimen was substantial, as 22% of patients had inadequate chemotherapy due to adverse drug reactions. In another study in which a consecutive sample of gold miners with pulmonary TB was allocated to receive rifampicin, isoniazid, pyrazinamide and ethambutol on weekdays for 5 months (8), silicotic patients with TB was found to have a higher incidence density for relapse, 1.55 (95% CI, 0.97–2.48) times those without silicosis. More research for better anti-TB regimen(s) in terms of efficacy and toxicity in the silicotic patients appears warranted.
What about the possibility of adjunctive host-directed therapy in silico-TB? Corticosteroid(s) exhibit a wide range of activities on the immune system of the host, covering both modulation of proinflammatory response and immunosuppression. However it is unlikely that the steroid congeners can have a distinct therapeutic role regarding TB in silicotic patients. Following the findings of earlier animal experiments, there has been increasing evidence in the recent past, regarding oxidative stress (and nitrosative stress) as additional orchestrating mechanisms in the pathogenesis of silicosis (9). Oxidative stress in concert with immunological dysfunction also probably increases the risk of TB reactivation in silicotic subjects. We have reasonably hypothesised earlier that the adverse TB treatment outcomes (including delayed bacteriological conversion, failure, relapse and death), associated with diabetes mellitus or HIV infection, might be contributed partially by oxidative stress, or more broadly a disturbance of redox homeostasis (10,11). Oxidative stress inherent to diabetes mellitus or HIV infection can potentially induce the formation of more Mycobacterium tuberculosis persisters with hibernating metabolism, resulting in tolerance/recalcitrance to the bactericidal activity of anti-TB drugs (10,11). Recently, in a laboratory study involving the treatment of M. tuberculosis clinical cultures with hydrogen peroxide, bacilli with the ultrastructural characteristics of dormant persisters, in terms of reduced cell envelope thickness and increased accumulation of intracytoplasmic lipid inclusions, were observed (12). These findings furnish evidence to support our biologically plausible hypothesis. In TB associated with silicosis, we strongly feel that a set of mechanisms similar to that for TB associated with diabetes mellitus or HIV applies (Figure 1). It appears that the afore-said hypothetical model would help in identifying research directions for TB in silicosis, and it is hoped that, in the future antioxidants (and beyond, especially therapeutics for tackling nitrosative stress) might emerge as potential adjuncts to the treatment of latent TB infection and TB disease in patients with silicosis.
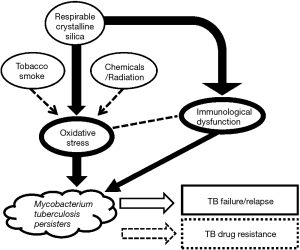
Additionally, chemoprophylaxis for latent TB infection should probably be considered among workers with prolonged silica exposure, whether or not silicosis has developed, as they are also at a high risk of having TB reactivation, especially in geographical regions with high prevalence of TB and HIV infection. The choice of preventive treatment regimen is influenced by the prevalence of drug-resistant TB in the local population and migrant workers. As such, latent TB treatment guidelines for subjects with silicosis or prolonged silica exposure may vary to some extent between countries or geographical regions with different TB epidemiological profiles.
Last but not least, aside from improving the working conditions of people exposed to silica, TB diagnosis and treatment facilities should be rendered with greater availability, accessibility and affordability for these workers, especially the miners.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Wing Wai Yew was consultant to Otsuka Pharmaceutical Company until July 2016. The other authors have no conflicts of interest to declare.
References
- Maeda M, Nishimura Y, Kumagai N, et al. Dysregulation of the immune system caused by silica and asbestos. J Immunotoxicol 2010;7:268-78. [Crossref] [PubMed]
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015;46:1563-76. [Crossref] [PubMed]
- A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36-41. [Crossref] [PubMed]
- Cowie RL. Short course chemoprophylaxis with rifampicin, isoniazid and pyrazinamide for tuberculosis evaluated in gold miners with chronic silicosis: a double-blind placebo controlled trial. Tuber Lung Dis 1996;77:239-43. [Crossref] [PubMed]
- Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med 2014;370:301-10. [Crossref] [PubMed]
- Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011;365:2155-66. [Crossref] [PubMed]
- A controlled clinical comparison of 6 and 8 months of antituberculosis chemotherapy in the treatment of patients with silicotuberculosis in Hong Kong. Am Rev Respir Dis 1991;143:262-7. [Crossref] [PubMed]
- Cowie RL. Silicotuberculosis: long-term outcome after short-course chemotherapy. Tuber Lung Dis 1995;76:39-42. [Crossref] [PubMed]
- Lopes-Pacheco M, Bandeira E, Morales MM. Cell-Based Therapy for Silicosis. Stem Cells Int 2016;2016:5091838. [Crossref] [PubMed]
- Yew WW, Leung CC, Zhang Y. Oxidative stress and TB outcomes in patients with diabetes mellitus? J Antimicrob Chemother 2017;72:1552-5. [Crossref] [PubMed]
- Yew WW, Chan DP, Singhal A, et al. Does oxidative stress contribute to adverse outcomes in HIV-associated TB? J Antimicrob Chemother 2018;73:1117-20. [Crossref] [PubMed]
- Vijay S, Hai HT, Thu DDA, et al. Ultrastructural analysis of cell envelope and accumulation of lipid inclusions in clinical Mycobacterium tuberculosis isolates from sputum, oxidative stress, and iron deficiency. Front Microbiol 2018;8:2681. [Crossref] [PubMed]


