|
Review Article
Photodynamic therapy for the treatment of non-small cell lung cancer
Charles B Simone, II1, Joseph S Friedberg2, Eli Glatstein1, James P Stevenson3, Daniel H Sterman4, Stephen M Hahn1, Keith A Cengel1
Hospital of the University of Pennsylvania, Philadelphia, PA, USA, 1Department of Radiation Oncology; 2Department of Surgery, Division of Thoracic Surgery; 3Department of Medicine, Division of Medical Oncology; 4Department of Medicine, Division of Pulmonary, Allergy and Critical Care
Correspondence: Charles B. Simone, II, MD. Hospital of the University of Pennsylvania, Department of Radiation Oncology, Perelman Center for Advanced Medicine, TRC 2 West, 3400 Civic Center Blvd, Philadelphia, PA 19104. Tel: (215)-662-3998; Fax: (215)-349-8975. Email: charles.simone@uphs.upenn.edu.
|
|
Abstract
Photodynamic therapy is increasingly being utilized to treat thoracic malignancies. For patients with early-stage non-small cell lung cancer, photodynamic therapy is primarily employed as an endobronchial therapy to definitely treat endobronchial, roentgenographically occult, or synchronous primary carcinomas. As definitive monotherapy, photodynamic therapy is most effective in treating bronchoscopically visible lung cancers ≤1 cm with no extracartilaginous invasion. For patients with advanced-stage non-small cell lung cancer, photodynamic therapy can be used to palliate obstructing endobronchial lesions, as a component of definitive multi-modality therapy, or to increase operability or reduce the extent of operation required. A review of the available medical literature detailing all published studies utilizing photodynamic therapy to treat at least 10 patients with non-small cell lung cancer is performed, and treatment recommendations and summaries for photodynamic therapy applications are described.
Key words
Photodynamic therapy; lung cancer; endobronchial; palliative care; non-small cell lung cancer
J Thorac Dis 2012;4:63-75. DOI: 10.3978/j.issn.2072-1439.2011.11.05
|
|
Introduction
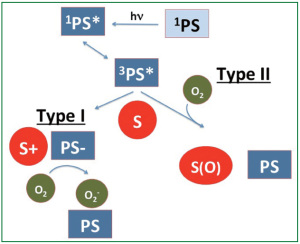
A projected 1.6 million people will be diagnosed worldwide with lung cancer in 2011. Despite standard lung cancer treatment modalities, including surgery, chemotherapy, and radiation therapy, lung cancer is the leading cause of death attributed to malignancies in the world, with nearly 1.4 million annual deaths ( 1). With the significant magnitude and severity of this disease, additional therapeutic and palliative treatment modalities have emerged. Photodynamic therapy (PDT) is an emerging treatment modality that is increasingly being utilized to treat thoracic malignancies. PDT employs a photosensitizing agent that is activated by light of a specific wavelength to produce reactive singlet oxygen ( 1O 2) that mediates cellular cytotoxicity. The mechanism of action of photodynamic therapy has been previously described ( 2) and is depicted in Figure 1. PDT works through direct cell killing via apoptosis or necrosis and by damaging tumor vasculature, and it may induce an inflammatory reaction that may lead to a host anti-tumor immune response. Following the administration of a photosensitizing agent, tumor loci are irradiated with appropriate wavelength light from a laser or other sources. A common light source for PDT is a laser that can be directed through fiberoptic cables and inserted through an endoscope into the lungs to treat bronchogenic malignancies ( 3- 5). For pleural PDT, light can be delivered by multiple methods, including microlenses and custom designed applicators ( 6). Porfimer sodium, the photosensitizing agent most commonly utilized to treat patients with thoracic malignancies, has been approved by the U.S. Food and Drug Administration to treat non-small cell lung cancer in patients for whom standard therapies are not appropriate and to palliate symptoms in patients with airway obstruction ( 7) ( Table 1). As the light needed to activate most photosensitizers typically cannot pass through more than 5-10 mm of tissue, PDT is usually applied to treat lung cancers involving the lining of internal organs or cavities, and it is less effective in treating larger tumors without prior surgical debulking or interstitially placed light sources ( 3- 6).
|
|
|
| Table 1. Photodynamic Therapy Photosensitizers Approved to Treat Lung Carcinoma. |
| Photosensitizer |
Wavelength |
Approval |
Pending Approval |
| Porfimer sodium (Photofrin) |
630 nm |
US, Europe, Asia |
|
| Temoporfin (m-tetrahydroxyphenylchlorin, Foscan) |
652 nm |
Europe |
US trials ongoing |
| Talaporfin (mono-(L)-aspartylchlorin-e6, NPe6, LS11) |
664 nm |
Asia |
US trials ongoing |
| 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) |
665 nm |
|
US trials ongoing |
|
Since the U.S. Food and Drug Administration approved PDT for the treatment of microinvasive endobronchial non-small cell lung cancer in early 1998 and advanced partially obstructing endobronchial lung cancer in late 1998 ( 7), there has been increasing interest in using PDT alone or in combination with standard therapeutic or palliative modalities to treat non-small cell lung cancer. Although there have been several recently published trials assessing the role of PDT alone or as part of multi-disciplinary therapy to treat non-small cell lung cancer, no recent review article has been published examining the utility of PDT to treat lung cancer. This review reports on those newer studies published utilizing PDT to treat lung cancer and serves as the most comprehensive review of PDT for non-small cell lung cancer to date by thoroughly detailing the existing medical literature for roentgenographically occult, early-stage, and endobronchial non-small cell lung cancer, synchronous primary bronchogenic carcinomas, and advanced-stage non-small cell lung cancer in an attempt to discern treatment recommendations and summaries for PDT applications and its multi-disciplinary utilization for these cancers.
|
|
Materials and Methods
MEDLINE and CANCERLIT databases were searched using key words “photodynamic” or “PDT” and “lung”, “thoracic”, “bronchogenic”, “endobronchial”, “palliative”, “pleural”, or “non-small cell”. All studies reporting on the use of photodynamic therapy to treat human subjects were indiscriminately reviewed, regardless of date published or language of publication. Studies reporting on fewer than 10 patients were not included in this analysis unless they substantially advanced the available literature discussion for the disease topic described. Additionally, when authors published findings in multiple sources, the most recent publication was included. Older publications were also included if they reported on patient subsets that were not better detailed in the most recent publications.
|
|
Non-small Cell Lung Cancer overview
The use of PDT for both early-stage or advanced non-small cell lung cancer has several advantages over other therapies. PDT has a relatively favorable side effect profile when compared to other treatment modalities like surgery, external beam radiation therapy, or endobronchial brachytherapy. PDT administration is typically performed on an outpatient basis. Patients can be retreated with PDT for incomplete disease response or subsequent disease recurrence, and PDT does not typically compromise future therapeutic options in the event that additional treatment modalities become necessary. Additionally, mutations that confer resistance to chemotherapy or radiation therapy do not limit the efficacy of PDT. Despite these advantages, limitations of PDT include patient photosensitivity following therapy, bronchogenic tumors that invade the pulmonary artery are not amenable to therapy, and the treatment of thoracic malignancies with PDT is dependent on tumor location and size.
Following the initial approval by the U.S. Food and Drug Administration to use PDT to treat patients with non-small cell lung cancer, the therapeutic applications of PDT for bronchogenic carcinomas have increased ( Table 2). For early-stage disease, PDT is primarily employed as an endobronchial therapy to definitively treat endobronchial or roentgenographically occult tumors. Similarly, patients with multiple primary lung cancers may be definitively treated with PDT. For advanced-stage or metastatic disease, PDT is primarily employed to palliate symptomatic patients with obstructing endobronchial lesions who have airway compromise or hemoptysis. Patients with advanced disease may be treated with PDT in an attempt to increase operability or reduce the extent of operation required. Patients with pleural dissemination of non-small cell lung cancer may also be treated with intraoperative PDT following macroscopically complete surgical resection.
|
|
|
| Table 2. Published Clinical Indications of PDT to Treat Patients with Non-Small Cell Lung Cancer Thoracic Malignancies. |
| Definitive Therapy for Early-stage Central Endobronchial Tumors |
| Definitive Therapy for Early-stage Locally Recurrent Central Tumors Following Definitive Surgery or Radiation Therapy |
| Definitive Therapy for Roentgenographically Occult Central Tumors |
| Definitive Therapy for Synchronous Primary Carcinomas |
| Definitive Therapy for Early-stage Peripheral Lung Lesions |
| Palliation to Reduce Endobronchial luminal Obstruction and Tumor Stenosis, Improve Performance Status and Respiratory Function, and Resolve Acute Hemoptysis and Poststenotic Pneumonia |
| Neoadjuvant Therapy to Reduce the Extent of Surgical Resection (Pneumonectomy g Lobectomy) |
| Neoadjuvant Therapy to Convert Originally Inoperable Patients to Surgical Candidates |
| Treatment of Locally Advanced Disease as Part of Multi-modality Therapy |
| Treatment of Disease with Pleural Spread as Part of Multi-modality Therapy |
|
Since their early report of PDT administration to treat 13 patients with lung cancer ( 8), Investigators at Tokyo Medical College, Tokyo Medical University Hospital have been leaders in the use of PDT to treat patients with non-small cell lung cancer. These investigators have reported on the utility of PDT for each of the aforementioned indications for non-small cell lung cancer and have also pioneered PDT use for the treatment of peripheral lung lesions. A thorough review of their experience and the remainder the literature addressing the utilization of PDT to treat non-small cell lung cancer by clinical situation follows, along with summaries and recommendations. |
|
Advanced-stage Non-small Cell Lung Cancer
Advanced-stage and metastatic non-small cell lung cancer are associated with a grave prognosis, with a median survival of less than one year. Patients with advanced-stage lung cancer are often debilitated and have significant morbidity and decreased quality of life ( 9, 10). Palliative care, therefore, plays a prominent role in the care and management of these patients. The utility of palliative care in improving the quality of life for patients with advanced non-small cell lung cancer is now widely recognized. Several studies have reported an association between depressed mood and/or poor quality of life and shorter survival among patients with metastatic non-small cell lung cancer ( 11- 13). For patients with locally advanced or metastatic non-small cell lung cancer, PDT has been most commonly utilized to palliate symptoms. Endobronchial PDT can decrease airway obstruction and allow for improved respiratory function and quality of life ( 14). PDT can also be used to palliative patients with uncontrollable life-threatening hemoptysis. In this manner, PDT can cause thrombosis and control small vessel bleeding from a variety of locations and etiologies ( 15). One of the earlier reports on PDT for palliation for lung cancer describes its utilization to treat 15 consecutive patients with advanced squamous bronchogenic carcinomas in London, in which all tumors responded and symptomatic relief was achieved ( 16). PDT was also used to successfully palliate symptoms among seven patients with thoracic metastases and three primary lung cancers at the National Cancer Institute ( 17). It should be noted, however, that early studies reporting on PDT to treat advanced lung cancer found this modality to be less successful in controlling disease than when used for other primary cancer sites. Of the variety of tumors treated with PDT in Beijing from 1982-1985, the complete response rate for lung cancer lesions (n=54) was significantly lower than the remaining 486 lesions (17% vs. 45%). The overall response rate for patients with lung cancer, however, was 89% ( 18). Investigators from the University of California, Irvine Medical Center similarly found five lung cancer patients to be less likely to achieve complete responses when compared to 72 patients with head and neck and breast malignancies treated with PDT between 1981-1983 ( 19). Additionally, among the 26 patients with inoperable non-small cell lung cancer treated with PDT at the Netherlands Cancer Institute, 10 of 11 patients with stage I disease achieved a complete response. However, among the 15 patients with stage III disease, all of whom had previously received external beam radiation therapy, brachytherapy, or Nd:YAG laser therapy, 11 patients had a partial response and four had tumor progression and died of pulmonary hemorrhages within six months of receiving PDT ( 20). PDT was also administered at the National Cancer Center Hospital in Japan to 28 patients with early lung cancer and 38 patients with advanced lung cancer having 69 unresectable malignant lesions of the trachea and bronchus. Patients were not suitable for radical treatment with chemotherapy or radiation therapy. Most patients achieve a complete response (13%), partial response (64%), or tumor regression (19%), whereas disease progression was only seen in 4% ( 21). Despite these difficulties, several studies have demonstrated the ability of PDT to successfully palliate patients with symptomatic locally-advanced non-small cell lung cancer. Among 10 patients with surgically unresectable advanced endobronchial obstructing non-small cell lung cancer treated with PDT by Northwestern University Medical School investigators from 1985-1989, PDT reduced pulmonary obstruction from 86% +/-2% to 57% +/-3%, and all patients had a decrease in symptoms ( 22). A study in the United Kingdom prospectively evaluated PDT as palliation for 100 patients with stage IIIa-IV advanced inoperable bronchogenic carcinoma with endobronchial luminal obstruction, of which 90% had non-small cell lung cancer and 82% had previously received chemotherapy or radiation therapy. The mean endoluminal obstruction fell from 85.8% to 17.5% following PDT, with similar improvements in mean forced vital capacity (FVC) and forced expiratory volume in one second (FEV1). The cohort achieved a 9-month mean survival and had a 19% 2-year overall survival ( 23). Russian investigators also demonstrated that PDT reduced bronchial obstruction among patients with stage IIIb-IV disease in 75% of cases (9/12) and achieved a 100% complete response rate for early-stage cancers (8/8), although two patients developed recurrent disease within 3 months of PDT ( 24). When University of California, Irvine investigators reviewed their first 10 patients with stage III-IV obstructive inoperable non-small cell lung cancer with hemoptysis, dyspnea, and airway obstruction treated with PDT, they found all 10 patients had a response to therapy and reduction in airway obstruction, and seven patients had resolution of acute hemoptysis ( 25). At the University of Alabama Hospital, 133 symptomatic patients were treated with PDT for endobronchial lung lesions of varying histologies, the majority of which were non-small cell lung cancer (n=89). PDT allowed for significant improvements in dyspnea in 74% of patients as assessed by the Modified Medical Research Council Dyspnea Scale ( 26). PDT may also improve the efficacy of surgery or allow for surgical resection among patients with locally-advanced non-small cell lung cancer. Based on the 191 patients treated by Russian investigators with endotracheobronchial surgeries, including 153 patients with advanced non-small cell lung cancer causing respiratory obstruction, the addition of PDT to surgery may improve patient outcomes when compared with surgery alone ( 27). PDT has also been successfully employed as a means to reduce the extent of resection by allowing patients with non-small cell lung cancer who were initially planned to undergo pneumonectomy the ability to undergo lobectomy ( 28, 29), or to convert originally inoperable patients to surgical candidates ( 29). Of the 26 patients with various stages of non-small cell lung cancer that received preoperative PDT at Tokyo Medical University Hospital to reduce the extent of resection or convert inoperable disease to operable status, surgical goals were achieved in 85% of patients treated. Four of five originally inoperable patients were converted to resectable and 18 of 21 patients who were originally candidates only for pneumonectomy were able to undergo lobectomy ( 29). As PDT is based upon a photochemical reaction that is limited by the availability of molecular oxygen in the target tissue, 30 patients in Austria with inoperable non-small cell bronchogenic carcinoma and endobronchial stenosis were enrolled in a prospective pilot study from 1997-1999 assessing the combination of PDT with hyperbaric oxygen. Following PDT, without any therapy-related complications, patients reported significant improvements in dyspnea, hemoptysis, and poststenotic pneumonia (all P<0.05), and they had reductions in tumor stenosis (P<0.05) and improvements in performance status (P<0.05) ( 30). As with malignant pleural mesothelioma, PDT may be utilized as part of multi-modality management for patients with non-small cell lung cancer with pleural spread. A phase II trial enrolling 22 patients with pleural spread and clinical T4 non-small cell lung cancer was conducted at the University of Pennsylvania. Patients underwent surgery with complete (n=17) or partial tumor debulking (n=3), followed by hemithoracic pleural PDT (n=20) or PDT alone (n=2). The rate of 6-month local control for the cohort was 73.3% and the median overall survival was 21.7 months, compared with 6-9 months for similar patients based on historical controls ( 31). Summary: Patients with advanced-stage and metastatic non-small cell lung cancer have a grave prognosis and often require palliative interventions for symptomatic management. Although these patients are less likely to achieve a complete response to therapy than those with early-stage bronchogenic carcinomas, tumor response rates to PDT for patients with advanced bronchogenic carcinomas are still high and PDT is effective in palliating this patient population. PDT can reduce endobronchial luminal obstruction and tumor stenosis, allowing for improvements in patient dyspnea and pulmonary function. PDT can also resolve acute hemoptysis and poststenotic pneumonia and improve patient performance status. Furthermore, when used in the neoadjuvant setting, PDT can increase operability or reduce the extent of operation required. With limited available data, PDT also appears to be an effective component of multi-modality therapy for patients with locally advanced disease and may prolong survival for patients with non-small cell lung cancer with pleural spread when included as a component of multi-modality therapy.
PDT vs. Nd:YAG Laser Therapy for Advanced-stage Non-small Cell Lung Cancer
Patients with non-small cell lung cancer with symptomatic central airway obstruction often require palliative intervention, particularly for life-threatening obstruction or bleeding. Endobronchial treatment is typically associated with a rapid relief of symptoms and limited side effects. Central airway obstruction may be endoluminal, extraluminal, or infiltrating, and treatment should be tailored to the type of obstruction identified. Endoluminal obstruction is amenable to treatment with external beam radiation therapy, PDT, laser therapy, or brachytherapy ( 32, 33). Treatment recommendations for extraluminal obstructions and detailed discussions of radiation therapy and laser therapy are beyond the scope of this review. Nd:YAG laser therapy has historically been the most widely utilized modality to achieve tumor ablation within the tracheobronchial tree. As PDT may offer a safer and easier to perform alternative treatment ( 34), thermal ablation has been compared to PDT in an attempt to identify the optimal treatment modality for patients with obstructing bronchogenic carcinomas. One of the earliest reports comparing PDT with Nd:YAG laser therapy for palliation of patients with symptomatic non-small cell lung cancer was a retrospective study performed by Bulgarian investigators. They reported that a complete response was achieved in 58% of the 12 patients with central bronchial carcinoma patients treated with PDT, compared with 42% of 12 similar patients treated with Nd:YAG laser therapy ( 35). Of the 258 advanced lung carcinoma lesions causing endobronchial stenosis or obstruction treated with PDT (n=81) or Nd:YAG laser treatment (n=177) at Tokyo Medical University in the 1980’s and 1990’s, the overall treatment effectiveness was 75% with PDT and 81% with Nd:YAG laser. Nd:YAG laser was somewhat more effective for tumors in the trachea or main bronchi (93% vs. 73%) but not for tumors in lobar or segmental bronchi (73% vs. 76%). PDT, however, had an improved safety profile. No major complications occurred in patients receiving PDT, whereas Nd:YAG resulted in massive bleeding in 6%, perforations in 3%, and a procedural mortality rate of 1.7% ( 36). In contrast, University of Louisville School of Medicine investigators retrospectively compared treatment side effect profiles for 102 patients with bronchial obstruction treated from 1988-1999 with PDT (n=19) or Nd:YAG laser therapy (n=83) and found no differences in morbidity or mortality rates between the groups ( 34). In a retrospective review of 75 patients with obstructing primary lung cancer with airway obstruction or hemoptysis who were not surgical candidates treated from 1994-2002 at Allegheny General Hospital, no differences in patient or tumor characteristics were found between patients treating with varying therapeutic modalities or combinations of modalities that included Nd:YAG laser therapy, PDT, stenting, and endoluminal brachytherapy. The type of modality used in patients receiving a single type of intervention had no impact on survival. When compared with patients treated with a single type of intervention, patients receiving multiple treatment modalities had similar 1-year (50% vs. 51%) but significantly improved 3-year (22% vs. 2%, P=0.04) cumulative survival rates without increased complication rates ( 37). Spanish investigators performed a prospective randomized controlled trial comparing PDT (n=14) to Nd-YAG laser therapy (n=17) in 31 patients with partial or complete tracheobronchial obstruction due to inoperable non-small cell lung cancer. Although patients in both groups experienced symptomatic relief after treatment (P=0.003), patients receiving PDT experienced a significantly longer time until treatment failure (P=0.03) and longer median survival (P=0.007) ( 38). A recent systematic review was conducted to evaluate the clinical effectiveness and safety of PDT in the treatment of a variety of cancers and precancerous conditions. Among the 88 trials reported in 141 publications that were analyzed, only seven trials of 329 patients investigated PDT for lung cancer. Only five of these trials were published, and no trials were more recent than 2002. Despite this, the authors concluded that two trials suggested that PDT plus radiation therapy may be more effective than radiation therapy alone in the palliative treatment of non-small cell lung cancer. However, the authors concluded that three trials do not answer if PDT is superior, equivalent, or inferior to Nd:YAG ( 39). Summary: With limited comparative data available, PDT and Nd:YAG laser therapy appear to be equally effective in relieving intraluminal obstruction by tumor and palliating patient symptoms. Although Nd:YAG therapy does not result in subsequent photosensitivity, PDT is perhaps easier to perform, does not require general anesthesia, and may have a somewhat better safety profile. PDT may allow for a longer time to treatment failure. The use of each modality should be tailed to the clinical situation and may be dependent on practitioner preference and experience. The combined use of these modalities or the use of each modality in conjunction with another local therapy, such as external beam radiation therapy or endobronchial brachytherapy, may allow for improved local control or a longer duration of symptomatic control at the expense of increased treatment toxicity.
PDT in Combination with Radiation Therapy, Chemotherapy for Advanced-stage Non-small Cell Lung Cancer
External beam radiation therapy and endobronchial brachytherapy are two of the most established and effective palliative treatment modalities for patients with central obstruction from non-small cell lung cancer. The role of brachytherapy in this setting may optimally be reserved for patients who have failed a prior course of external beam radiation therapy ( 33). The addition of external beam radiation therapy to endobronchial brachytherapy can, in rare cases, can be associated with potentially fatal massive hemoptysis, formation of tracheoesophageal fistulas, radiation-induced bronchitis, bronchial stenosis, and bronchospasms ( 40). The administration of PDT following external beam radiation therapy may have a more favorable toxicity profile than the administration of endobronchial brachytherapy and may be a viable treatment option as part of multi-modality therapy for patients with symptomatic obstructive non-small cell lung cancer. Although very limited in patient numbers (n=11), Vancouver investigators completed a randomized trial to determine if PDT improves outcomes when combined with external beam radiation therapy to 30 Gy in 10 fractions over two weeks for patients with inoperable bronchogenic non-small cell carcinoma obstructing a central airway. All patients achieved symptomatic improvement and objective evidence of tumor regression at four weeks following therapy. However, at longer follow-up, based on post-treatment spirometry, ventilation-perfusion lung scans, CT scans, bronchoscopies, and quality of life scores, the addition of PDT prior to radiation therapy provided significantly improved and more durable local control ( 41). Additionally, 41 patients with locally advanced non-small cell lung cancer, including 78% with stage III disease, received induction PDT and chemotherapy and/or radiation therapy at Ohio State University Medical Center. PDT-based induction allowed 57% of patients initially deemed unresectable to undergo definitive surgical resection and 27% initially deemed in need of pneumonectomy to undergo lobectomy, pathological downstaging in 64%, and a pathologic complete response in 18% undergoing surgery. Overall, 46% of patients were alive at 3 years following therapy, and the mean survival was greatest in patients undergoing lobectomy (35.9 months) and shortest in those unable to undergo surgery after multi-modality therapy (14.7 months) ( 42). Prospective studies have also suggesting a possible synergistic effect when combining PDT and ionizing radiation, particularly brachytherapy. Among 32 patients with bulky endobronchial non-small cell bronchogenic carcinoma (range 10-60 mm along the bronchus) treated at Beth Israel Deaconess Medical Center with PDT followed six weeks later with brachytherapy (Iridium-192, 4 Gy weekly for five weeks), patients achieved 81% local control, 94% distant metastasis-free survival, and 100% overall survival at a mean follow-up of 24 months. There were no severe complications, including hemoptysis, fistula formation, post-obstructive pneumonia, or obstructive bronchial scarring requiring intervention ( 43). Brody School of Medicine at East Carolina University investigators also safely combined PDT with high-dose-rate brachytherapy for patients with symptomatic obstruction from endobronchial non-small cell lung cancer ( 44). However, patients with upper aerodigestive tract carcinomas who previously received treatment with both external beam radiation therapy and intraluminal brachytherapy may be at higher risk for complications when subsequently treated with PDT ( 45). Summary: PDT may be most effective when delivered as part of multi-modality therapy. The combination of PDT with external beam radiation therapy or endobronchial brachytherapy may have a more favorable safety profile than the combination of external beam radiation therapy with endobronchial brachytherapy. The addition of PDT to external beam radiation therapy or endobronchial brachytherapy may significantly improve symptomatic relief and lengthen the duration of local control among patients with symptomatic obstructing non-small cell lung cancer. Although the optimal sequence for combined therapy is undetermined, local control may be best when high-dose-rate brachytherapy is administered prior to PDT and when PDT is delivered prior to external beam radiation therapy. Tumor control may be optimized when PDT and radiation therapy are delivered within one month of brachytherapy therapy ( 44). PDT may also be administered as induction therapy before chemotherapy and can increase tumor resectability and decrease the extent of surgery required when delivered as part of multi-modality therapy.
|
|
Early-stage Non-small Cell Lung Cancer
Roentgenographically Occult Bronchogenic Non-small Cell Lung Cancer
Even with improvements in imaging diagnostics over the past two decades, a subset of patients with bronchoscopically-confirmed pulmonary carcinomas has lesions that are undetectable by currently available imaging technologies. Such lesions are termed roentgenographically occult. Roentgenographically occult carcinomas can be detected in high-risk patient groups that undergo sputum cytology screening. Patients with roentgenographically occult carcinomas most often present with early-stage squamous cell carcinoma with centrally-located disease.
Surgical resection has historically been the therapy of choice for patients with such lesions but can be associated with significant morbidity. As fewer than 1% of patients with roentgenographically occult bronchogenic carcinoma that are endoscopically visibility but lack cartilaginous invasion have metastatic lymph node involvement, focal therapy to the primary lesion alone can be curative ( 46). PDT and bronchoscopic electrocautery, using an insulated flexible bronchoscope to coagulate and vaporize tumor, have emerged as definitive treatment options for such patients ( 47). The latter modality is beyond the scope of this review. One of the earlier reports of PDT to treat roentgenographically occult bronchogenic carcinoma was performed by National Kinki Central Hospital for Chest Diseases investigators. Between 1983-1990, they treated 25 patients with 29 roentgenographically occult lung carcinomas with PDT. Complete remission was achieved in 72% of lesions, including 89% (17/19) of lesions ≤1 cm and 86% (18/21) of visible peripheral area lesions. Among patients with a compete response, five developed disease recurrence within the first two years following PDT, with all relapses occurring at least seven months following initial therapy ( 48). At the National Cancer Center Hospital in Tokyo, 36 patients with 39 roentgenographically occult lung cancer lesions of the trachea and bronchus were treated with PDT through a fiberoptic bronchoscope between 1981-1987. PDT was not associated with any significant toxicity. All patients had a clinical response to therapy, and a complete response was achieved in 31% of lesions. Patients with a partial response received subsequent surgery or radiation therapy. At a mean follow-up of 5.4 years, 43% of patients were alive with no apparent recurrence or metastasis ( 49). In a pulled series from five Japanese hospitals, 33 patients with 40 roentgenographically occult carcinomas proximal to a subsegmental bronchus were treated with PDT by an excimer dye laser via flexible bronchoscope. WHO grade >2 toxicity occurred in 8% of patients and included transient dermatitis and obstructive pneumonitis. A complete response to PDT was achieved in all lesions ≤1 cm (n=32) but in only three of eight larger carcinomas ( 50). From 1986-1992, 39 roentgenologically occult predominantly squamous cell (38/39) lung cancers in 29 patients were treated at Osaka Prefectural Habikino Hospital in Japan using PDT, with thoracic radiation therapy administered for patients with residual tumor following PDT. Initial PDT achieved a complete response in 64% of lesions. A complete response to PDT was more likely for lesions that were superficially infiltrating than those that were nodular in appearance (76% vs. 43%). Of the 14 lesions not completely responsive to PDT, 71% achieved a subsequent complete response to radiation therapy. All subsequent recurrences during the follow-up period (n=9) were retreated with PDT and/or radiation therapy. The 2-year, 3-year, and 5-year overall survival rates for the cohort were 93%, 72%, and 56%, respectively ( 51). Additionally, Tohoku University Hospital investigators treated 48 medically operable patients with roentgenographically occult bronchogenic squamous cell carcinomas with bronchoscopic longitudinal tumor lengths of ≤10 mm with PDT from 1994-2006. A complete response was achieved in 94% of patients, and the 5-year and 10-year overall survival rates for the cohort were 81% and 71%, respectively ( 52). A cohort of 32 medically inoperable patients with intraluminal central airway microinvasive radiographically occult lung cancer with lesions ≤1 cm were treated at Vrije Universiteit Medical Center in Amsterdam with bronchoscopic therapy that included PDT, Nd:YAG laser therapy, electrocautery, or argon plasma coagulation. No differences in patient outcomes were observed based on the type of bronchoscopic therapy administered, and the 5-year overall survival for the cohort was 50% ( 53). Summary: While surgical resection is an effective treatment modality for roentgenologically occult bronchogenic carcinomas, PDT is minimally invasive, is associated with less treatment morbidity, and has a high initial complete response rate to therapy. Even among surgical candidates, therefore, PDT may be considered a first-line treatment modality for patients with roentgenologically occult squamous cell carcinomas of the lung that are bronchoscopically visible, have tumor lengths of ≤1 cm, have no extracartilaginous invasion or lymph node involvement, and have no radiologic findings on high resolution computed tomography imaging ( 46). However, patients undergoing PDT may be at higher risk for local recurrence and should be carefully monitored. Most recurrences following PDT can be adequately treated with surgery, radiation therapy, and repeat administration of PDT.
Early-stage and Endobronchial Non-small Cell Lung Cancer
Similar to roentgenologically occult bronchogenic carcinomas, early-stage and curable endobronchial non-small cell carcinomas can be effectively treated with PDT. Reports from Mayo Clinic investigators provided early support for the use of PDT to treat patients with early centrally located bronchogenic carcinomas. Their 1982 report of 10 patients with tracheobronchial tree carcinomas treated with laser phototherapy administered via flexible fiberbronchoscope demonstrated that complete tumor responses were only obtained for patients with microinvasive tumors with depths of invasion less than 5 mm ( 54). Following this report, there has been increasing interest in the utilization of PDT to treat thoracic malignancies. When reporting on their expanded cohort of 38 patients with 40 carcinomas treated from 1980-1986, a complete response was achieved in 35% of lesions, all of which were ≤3 cm 2 in surface area. In fact, a complete response was achieved in 48% of lesions ≤3 cm 2 in surface area (n=29) but none of the 11 lesions >3 cm 2 ( 55). Although initially and still most typically utilized for patients unsuitable for surgical resection, PDT has became an established alternative treatment modality to surgery for patients with small, early-stage central non-small cell lung cancers. Mayo Clinic investigators further reported that among 21 surgical candidates with 23 early superficial squamous cell carcinomas who declined resection, PDT alone allowed for a complete response in 70% of lesions, with 75% of these responses sustained for at least 12 months. Overall, 48% of patients subsequently required surgery for persistent disease, disease recurrence, or a second primary lung malignancy, leading the authors to conclude that at least 22% of patients with early-stage squamous cell lung cancer who are candidates for surgery can be treated with PDT and spared invasive resection ( 56). In a related cost-effectiveness analysis assessing monetary expenses and quality adjusted life years saved for early-stage lung cancer without lymph node metastasis, the total cost of PDT was found to be 43% less expensive than lobectomy, and the cost-effectiveness rate for patients undergoing surgery was 1.3-1.5 times higher ( 57). Other early reports in the 1980’s also demonstrated excellent tumor responses to PDT. A complete response was achieved in all but 1 of the 35 patients with primary non-small cell lung cancer (n=29), other primary pulmonary tumors (n=3), and metastatic pulmonary lesions (n=3) within the tracheobronchial tree treated in Los Angeles with PDT ( 58). Independent early reports from China indicated that PDT achieved a 96% response rate among 21 patients with 24 lung cancer lesions ( 59) and a 97% response rate among 54 patients with 69 cancer foci in the lumen of the trachea-bronchus ( 60), with both groups reporting no severe complications from therapy. The Japan Lung Cancer Photodynamic Therapy Study Group conducted a phase II study from 1989-1992 in which patients were treated with PDT with porfimer sodium as photosensitizer for centrally located subsegmental or larger bronchi early-stage lung cancer. PDT toxicities included transient elevation of ALT (1.9%), pulmonary toxicity (7.7%), allergic reaction (7.7%), and sunburn (1.9%). Of 49 patients with 59 carcinomas assessable for response, a complete response was achieved in 85% of lesions, and the median duration of complete response was greater than 14 months. A complete response was significantly more likely for lesions with a longitudinal tumor extent of ≤1 cm (n=45) than for those >1 cm (n=14) (98% vs. 43%, P=0.00001) ( 61). Additionally, 50 patients with 59 early squamous cell carcinomas of the bronchus were treated at National Kinki Central Hospital for Chest Diseases with PDT from 1983-1997. A complete response was achieved in 73% of lesions. No patient recurred if they were disease free for the two years following PDT, and 53% of all lesions were considered cured with PDT. Bcl-2 immunoreactivity was detected in 23 tumors (47%) and p53 immunoreactivity was detected in 22 tumors (45%). Although bcl-2 had greater expression among larger than smaller tumors (P=0.0155), neither bcl-2 or p53 status were associated with local recurrence or long-term outcomes following PDT ( 62). From 1982-1996, 175 patients with varying stages of endobronchial non-small cell lung cancer were treated over a 14-year period with PDT at the Grant Laser Center in Ohio. PDT morbidity was minimal, and survival significantly differed by clinical stage (P<0.0001). Median survival for the 16 patients with stage I disease was not reached at the time of reporting but was 7 months for the entire cohort, suggesting that PDT can be an effective alternative treatment to surgery for patients with stage I carcinoma in whom the risk of surgery is high ( 63). Investigators in Hiroshima, Japan treated 18 cases of early-stage squamous cell carcinomas with PDT from 1997-2001. A complete response and long-term control was achieved in all nine lesions that were intracartilaginous at a median follow-up after PDT of 32 months. The remaining nine lesions with extracartilaginous extension were treated with combinations of surgery, PDT, radiation therapy, and chemotherapy, and all patients in this group were disease-free at a median follow-up of 24 months ( 64). Italian investigators also found a 62% complete response rate and 100% overall response rate when treating 23 patients with 26 early central bronchogenic squamous cell carcinomas with PDT ( 65). As reported by Egyptian and Japanese investigators, of 16 centrally located early lung carcinomas treated from 1999-2010 with PDT, a complete response was achieved in 88% of lesions, but 29% of lesions that completely responded were subsequently found to recur within 12 months of PDT administration ( 66). From 1980-1995, 240 patients with 283 central lung cancer lesions were treated at Tokyo Medical University Hospital. The overall response rate was 99%, with 40% of all lesions achieving a complete response. A complete response was achieved in 83% (79/95) of early-stage lesions, including in 94% (65/69) of lesions <1.0 cm, 54% (14/26) of lesions ≥1.0 cm, and 38% (6/16) of lesions ≥2.0 cm (P=0.00001). Among these early-stage cancers, lesions with a clearly visible distal tumor margin (n=67) were somewhat more likely to respond completely to PDT than tumors without a visible margin (n=28) [86.8% vs. 71.4%, P=0.09] ( 67). When these investigators evaluated PDT utilizing a YAG-OPO laser as the light source when treating 26 patients with 29 early-stage lung cancers with PDT from 1995-1996, the complete remission and overall response rates were 83% and 97%, respectively ( 68). A more recent update of the series from Tokyo Medical University Hospital demonstrated that among 93 patients treated with PDT for 114 central early-stage lung cancers with long-term follow-up, the complete response was higher for lesions <1.0 cm (77/83) than ≥1.0 cm (18/31) [93% vs. 58%, P<0.001]. The 5-year survival rate did not differ by tumor size (58% vs. 59%, P=0.207). Among lesions <1.0 cm with an initial complete response to PDT, 12% recurred. Recurrences with histologic low-to-moderate atypia could successfully be salvaged with additional PDT, whereas recurrences found to have high-grade atypia demonstrated intracartilaginous bronchial wall tumor invasion, potentially related to inadequate laser irradiation and penetration ( 69). In a recent review of original articles of >10 patients with early central lung cancer treated with PDT, 15 trials of 626 patients with 715 lesions were identified. PDT was typically employed for patient ineligibility for operation, and porfimer sodium photosensitizer followed by bronchoscopic illumination using a laser light after an interval of 48-72 hours was the most common treatment course. PDT-related death occurred in 1 patient (0.15%), whereas adverse events included photosensitivity skin reactions in 5-28%, respiratory complications in 0-18%, and non-fatal hemoptysis in 0-8%. A complete response was achieved in 30-100% of patients for 2-120 months. The overall 5-year survival rate was estimated to be 61% ( 70). PDT may also have an emerging role as salvage therapy for patients who develop tumor recurrence following surgical resection of early-stage non-small cell lung cancer ( 71). In fact, among the first 200 patients treated with bronchoscopic PDT by investigators at Yorkshire Laser Centre, 21 had early central lung cancer and were ineligible or declined surgery (n=11) or had metachronous (n=1) or recurrent (n=10) endobronchial lesions following surgical resection (n=6) or external beam radiation therapy and/or Nd:YAG laser therapy (n=4). No significant toxicities were encountered following PDT administration, and all 21 patients achieved a complete response to PDT. However, 33% of patients required subsequent repeat applications of PDT due to disease recurrence within 15 months of initial therapy ( 72). Additionally, 40 patients with early (T1N0M0) medically inoperable lesions (n=12) or recurrent carcinoma in situ following previous treatment for invasive lung cancer (n=28) were treated by Italian investigators with PDT from 1989-2004. PDT was not associated with any severe adverse events and achieved a 72% overall complete response rate, with similar complete response rates for Tis lesions and T1 lesions (73% vs. 69%, P>0.05). The median overall survival for the cohort was 91 months, with patients with Tis lesions demonstrating a longer median survival (120 months vs. 36 months, P=0.03) ( 73). Of note, recurrent tumors in the bronchial stump following initial therapy may be better served by a treatment modality other than PDT if there is difficulty in delivering light endobronchially to distal tissues ( 74). Summary: Early-stage and curable endobronchial non-small cell carcinomas can be effectively treated with PDT. PDT is most effective in patients with lesions ≤1 cm and no extracartilaginous invasion or lymph node involvement. Although most frequently administered in patients who are not medical or surgical candidates for definitive tumor resection, PDT has proven to be an effective treatment modality even among patients who are surgical candidates. In this patient population, PDT has a more favorable toxicity profile and is more cost effective. Such patients who undergo definitive PDT should be carefully monitored for local recurrence. PDT may also be employed as salvage therapy for patients who develop non-bronchial stump tumor recurrence following surgical resection of early-stage non-small cell lung cancer.
|
|
New Indications, Photosensitizers, and Surveillance for PDT Treatment of Early-stage Non-small Cell Lung Cancer
A novel form of delivery of PDT has recently been evaluated at Tokyo Medical University Hospital as definitive therapy for patients who are unfit for surgery or radiation therapy and who have peripheral lung cancers less than 1 cm in size. Following photosensitizer administration, needles containing an internal catheter were inserted percutaneously under CT guidance and then extracted. Light delivery then followed through a catheter positioned within the tumor. This technique was employed in nine patients, seven of whom achieved partial remission. The procedure, however, was complicated by two cases of pneumothorax ( 75). The use of second generation photosensitizers has also recently been employed in clinical trials in Japan. A phase II study of 41 patients at Tokyo Medical University Hospital to assess the use of mono-L-aspartyl chlorin e6 (NPe6, 40 mg/m 2, 664 nm), a second generation photosensitizer, for PDT (100 J/cm 2 four hours after photosensitizer) to treat early-stage central superficial lung squamous cell carcinoma lesions ≤2 cm. Skin photosensitivity disappeared within two weeks in 85% of patients and within 18 days in all patients, and no serious adverse drug reactions were observed. Additionally, 85% of patients achieved a complete response, prompting the authors to conclude NPe6 and diode laser will become a standard modality of PDT for central type early superficial squamous cell carcinoma of the lung ( 76). Thereafter, these investigators treated 75 additional patients with 91 centrally located early lung cancers with NPe6-based PDT from 2004-2008. Complete response rates were 94% for lesions ≤1.0 cm (n=70) and 90% for lesions >1.0 cm (n=21). The authors concluded that PDT with NPe6 photosensitizer produces effective tumor killing of large or deeply invasive tumors that have been reduced by electrocautery, whereas such lesions would not typically be amenable to adequate treatment with PDT employing porfimer sodium as photosensitizer ( 77). Improvements in endoscopy, imaging, and surveillance may allow for PDT to become more effective and more widely utilized. The cure rates for tumors ≤1 cm may exceed 90% but significantly decline for larger lesions. Newer imaging techniques, including fluorescence endoscopy, may better visualize tumor extension along the bronchial wall and may be able to prevent tumor undertreatment with PDT or improve surveillance following PDT administration. In fact, the addition of the newly developed video endoscopy-based auto fluorescence bronchoscope system (SAFE-3000) to conventional bronchoscopy increased the sensitivity of post-PDT surveillance from 69% to 100% ( 66). Furthermore, the addition of endobronchial ultrasonography to endoscopy can better assess tumor thickness and may also reduce tumor undertreatment with PDT. Additionally, improvements in imaging techniques may improve surveillance following PDT and increase the salvage rates among patients who recur following initial successful PDT administration. Summary: The utilization of newer and potentially improved photosensitizers may expand the clinical indications of PDT in the treatment of early-stage central non-small cell lung cancer and also allow for more effective treatment of larger or more invasive carcinomas. Newer imaging techniques, including fluorescence endoscopy, may better guide PDT therapy and also improve the sensitivity of surveillance following PDT administration. Improvements in post-PDT surveillance may result in higher salvage rates among patients who recur following PDT administration, which may lead to the more widespread utilization of PDT as definitive therapy for early central bronchogenic carcinomas. The treatment of peripheral lung lesions with PDT is still an unproven and experimental treatment modality and should not currently be considered a primary therapeutic option for patients unable to undergo surgical resection or external beam radiation therapy.
|
|
Synchronous Primary Bronchogenic Carcinomas
Synchronous multiple primary lung cancers occur in 1-15% of patients with lung malignancies, with the incidence rising due to improvements in computed tomography scanning and increasing utilization of positron emission tomography scanning ( 78). Patients with synchronous bronchogenic tumors typically present with centrally located disease that is most often of squamous cell histology ( 79). As the long-term survival for patients with synchronous primary lung tumors is superior to that for patients with stage III or IV disease for reasons other than synchronous cancers, aggressive management of each primary tumor is typically indicated, with surgical resection most often employed ( 80). PDT in the setting of synchronous intrathoracic primary tumors has typically been utilized among patients with significant medical comorbidities or poor performance status, those who are not otherwise curable with surgical intervention, and those who would be at significant risk of pulmonary morbidity from surgery or radiation therapy following definitive treatment of multiple tumors ( 81). PDT has even been reported to safe and effective in definitively treating synchronous quadruple lung primary malignancies ( 82). As a substantial number of patients with multiple lung cancers have a heavy tobacco smoking history and resulting limited pulmonary function, a treatment modality that spares normal tissues, such as PDT, warrants investigation. Among 13 patients with synchronous (n=5) or metachronous (n=8) central lung cancers treated at Tokyo Medical University Hospital form 1980-1989 with endoscopic PDT alone for each lesion (n=3) or PDT for the smaller lesions and surgery for the larger lesion (n=10), their median survival was 38 months (range 14-87 months) ( 83). Investigators at Tokyo Medical University Hospital later reported on 22 patients with centrally-located early lung cancers that were treated from 2004-2008 with PDT alone for each lesion (n=11) or surgery for more peripheral primary lung lesions and PDT for more centrally-located lesions (n=11). Of the 39 central tumors treated with PDT, a complete response was achieved in all lesions by two months following PDT, and all patients were alive with variable follow-up of up to five years ( 79). PDT was also found to be an effective treatment modality among the 44 patients with multiple primary lung carcinomas treated at Osaka University Hospital with surgery, PDT, radiation therapy, and/or chemotherapy ( 84). In the largest reported cohort of patients with synchronous lung primary malignancies treated with PDT, 104 patients with multiple tumors of the trachea and lobar and segmental bronchi underwent endoluminal endoscopic surgery and PDT. Outcomes were found to significantly depend on tumor size, with complete tumor regression occurring for all tumors less than 1 cm in diameter ( 85). Summary: Synchronous primary endobronchial carcinomas are an increasingly common occurrence. Despite the advanced stage of disease associated with synchronous tumors, long-term survival is possible and definitive management of each lesion is warranted. PDT should be considered for any patient with synchronous endobronchial carcinomas who is medically or surgically not a candidate for curative resection. As surgical resection or external beam radiation therapy to multiple lesions may result in significant risk of pulmonary morbidity, PDT may be considered in properly selected patients who are surgical candidates, particularly for patients with all tumors less than 1 cm in diameter.
|
|
Conclusions
PDT is a relatively new and promising anti-tumor treatment modality for patients with lung cancer and may currently be underutilized. PDT to treat non-small cell lung cancer is typically well tolerated and uncommonly associated with severe toxicities. PDT can be administered as definitive monotherapy for patients with early-stage non-small cell lung cancer who have central or roentgenographically occult carcinomas or synchronous primary endobronchial carcinomas. In this setting, PDT is most effective in patients with bronchoscopically visible lesions ≤1 cm with no extracartilaginous invasion. Close surveillance following definitive PDT to assess for local recurrence is warranted, however, particularly in the first two years following therapy. For advanced-stage non-small cell lung cancer, PDT can be used to palliate symptomatic patients with obstructing endobronchial lesions or as a component of definitive multi-modality therapy. PDT can also be administered neoadjuvantly in an attempt to increase operability or reduce the extent of operation required. Importantly, PDT has not been reported to produce second malignant neoplasms, and PDT typically does not compromise future treatment options for patients in need of additional definitive therapy with isolated endobronchial or other local recurrences or for those with progressive disease in needed of additional palliative therapy. The efficacy of PDT and subsequent post-therapy surveillance may be increased by newer photosensitizers and improved endoscopic and imaging techniques, which may lead to a more widespread utilization of PDT to treat early central bronchogenic carcinomas and lung malignancies.
|
|
Funding
This work was supported, in part, by National Institutes of Health, National Cancer Institute grant number P01 87971.
|
|
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.[LinkOut]
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011;61:250-81.[LinkOut]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889-905.[LinkOut]
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer 2003;3:380-7.[LinkOut]
- Vrouenraets MB, Visser GW, Snow GB, van Dongen GA. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res 2003;23:505-22.[LinkOut]
- Finlay JC, Zhu TC, Busch TM, Cengel K. Photodynamic therapy. Handbook of Biomedical Optics. In: DA Boas, C Pitris, N Ramanujam editors. Taylor & Francis Books Inc, 2011.
- Federal Drug Administration. “Medical Devices,” 19 July 2011. [Accessed Sep 9, 2011].[LinkOut]
- Hayata Y, Kato H, Konaka C, Ono J, Takizawa N. Hematoporphyrin derivative and laser photoradiation in the treatment of lung cancer. Chest 1982;81:269-77.[LinkOut]
- Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer 1995;71:633-6.[LinkOut]
- Lutz S, Norrell R, Bertucio C, Kachnic L, Johnson C, Arthur D, et al. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: a prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med 2001;4:157-65.[LinkOut]
- Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol 2005;23:6865-72.[LinkOut]
- Movsas B, Moughan J, Sarna L, Langer C, Werner-Wasik M, Nicolaou N, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol 2009;27:5816-22.[LinkOut]
- Pirl WF, Temel JS, Billings A, Dahlin C, Jackson V, Prigerson HG, et al.Depression after diagnosis of advanced non-small cell lung cancer and survival: a pilot study. Psychosomatics 2008;49:218-24.[LinkOut]
- Kato H, Konaka C, Saito M, Nishimiya K, Kawate N, Aizawa K, [Laser photodynamic therapy with hematoporphyrin derivative in lung cancer]. Nihon Geka Gakkai Zasshi 1985;86:1059-63.[LinkOut]
- McCaughan JS Jr, Hawley PC, LaRosa JC, Thomas JH, Hicks WJ. Photodynamic therapy to control life-threatening hemorrhage from hereditary hemorrhagic telangiectasia. Lasers Surg Med 1996;19:492-4.[LinkOut]
- Hugh-Jones P, Gardner WN. Laser photodynamic therapy for inoperable bronchogenic squamous carcinoma. Q J Med 1987;64:565-81.[LinkOut]
- Pass HI, Delaney T, Smith PD, Bonner R, Russo A. Bronchoscopic phototherapy at comparable dose rates: early results. Ann Thorac Surg 1989;47:693-9.[LinkOut]
- Li JH, Guo ZH, Jin ML, Zhao FY, Cai WM, Gao ML, et al. Photodynamic therapy in the treatment of malignant tumours: an analysis of 540 cases. J Photochem Photobiol B 1990;6:149-55.[LinkOut]
- Wile AG, Coffey J, Nahabedian MY, Baghdassarian R, Mason GR, Berns MW. Laser photoradiation therapy of cancer: an update of the experience at the University of California, Irvine. Lasers Surg Med 1984;4:5-12.[LinkOut]
- Sutedja T, Baas P, Stewart F, van Zandwijk N. A pilot study of photodynamic therapy in patients with inoperable non-small cell lung cancer. Eur J Cancer 1992;28A:1370-3.[LinkOut]
- Ono R, Egawa S, Ikeda S. [Combined treatment of endoscopic laser irradiation and radiotherapy in lung cancer]. Gan To Kagaku Ryoho 1989 Apr;16:1418-24.[LinkOut]
- LoCicero J 3rd, Metzdorff M, Almgren C. Photodynamic therapy in the palliation of late stage obstructing non-small cell lung cancer. Chest 1990;98:97-100.[LinkOut]
- Moghissi K, Dixon K, Stringer M, Freeman T, Thorpe A, Brown S. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15:1-6.[LinkOut]
- Iaitskiĭ NA, Gerasin VA, Orlov SV, Butenko AB, Molodtsova VP, Derevianko AV, et al. [Photodynamic therapy in treatment of lung cancer]. Vestn Khir Im I I Grek 2010;169:31-4.[LinkOut]
- Jones BU, Helmy M, Brenner M, Serna DL, Williams J, Chen JC, et al. Photodynamic therapy for patients with advanced non-small-cell carcinoma of the lung. Clin Lung Cancer 2001;3:37-41.[LinkOut]
- Minnich DJ, Bryant AS, Dooley A, Cerfolio RJ. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg 2010;89:1744-8.[LinkOut]
- Arsen'ev AI, Kanaev SV, Barchuk AS, Vedenin IaO, Klitenko VN, Gel'fond ML, et al. [Use of endotracheobronchial surgery in conjunction with radiochemotherapy for advanced non-small lung cancer]. Vopr Onkol 2007;53:461-7.[LinkOut]
- Konaka C, Usuda J, Kato H. [Preoperative photodynamic therapy for lung cancer]. Nihon Geka Gakkai Zasshi 2000 Jul;101:486-9.[LinkOut]
- Okunaka T, Hiyoshi T, Furukawa K, Yamamoto H, Tsuchida T, Usuda J, et al. Lung cancers treated with photodynamic therapy and surgery. Diagn Ther Endosc 1999;5:155-60.[LinkOut]
- Tomaselli F, Maier A, Sankin O, Anegg U, Stranzl U, Pinter H, et al. Acute effects of combined photodynamic therapy and hyperbaric oxygenation in lung cancer--a clinical pilot study. Lasers Surg Med 2001;28:399-403.[LinkOut]
- Friedberg JS, Mick R, Stevenson JP, Zhu T, Busch TM, Shin D, et al. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J Clin Oncol 2004;22:2192-201.[LinkOut]
- Stephens KE Jr, Wood DE. Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg 2000;119:289-96.[LinkOut]
- Cardona AF, Reveiz L, Ospina EG, Ospina V, Yepes A. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2008;(2):CD004284.[LinkOut]
- Taber SW, Buschemeyer WC 3rd, Fingar VH, Wieman TJ. The treatment of malignant endobronchial obstruction with laser ablation. Surgery 1999 Oct;126:730-3;discussion 733-5.[LinkOut]
- Karanov S, Kostadinov D, Shopova M, Kurtev P. Photodynamic therapy in lung and gastrointestinal cancers. J Photochem Photobiol B 1990;6:175-81.[LinkOut]
- Furukawa K, Okunaka T, Yamamoto H, Tsuchida T, Usuda J, Kumasaka H, et al. Effectiveness of Photodynamic Therapy and Nd-YAG Laser Treatment ftructed Tracheobronchial Malignancies. Diagn Ther Endosc 1999;5:161-6.[LinkOut]
- Santos RS, Raftopoulos Y, Keenan RJ, Halal A, Maley RH, Landreneau RJ. Bronchoscopic palliation of primary lung cancer: single or multimodality therapy? Surg Endosc 2004;18:931-6.[LinkOut]
- Diaz-Jiménez JP, Martínez-Ballarín JE, Llunell A, Farrero E, Rodríguez A, Castro MJ. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur Respir J 1999;14:800-5.[LinkOut]
- Fayter D, Corbett M, Heirs M, Fox D, Eastwood A. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett's oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol Assess 2010;14:1-288.[LinkOut]
- In: Perez CA, Brady LW, editors. Principles and Practice of Radiation Oncology, 2nd Edition. Philadelphia: JB Lippincott Co. 1992;50–63:114–23.
- Lam S, Kostashuk EC, Coy EP, Laukkanen E, LeRiche JC, Mueller HA, et al. A randomized comparative study of the safety and efficacy of photodynamic therapy using Photofrin II combined with palliative radiotherapy versus palliative radiotherapy alone in patients with inoperable obstructive non-small cell bronchogenic carcinoma. Photochem Photobiol 1987;46:893-7.[LinkOut]
- Ross P Jr, Grecula J, Bekaii-Saab T, Villalona-Calero M, Otterson G, Magro C. Incorporation of photodynamic therapy as an induction modality in non-small cell lung cancer. Lasers Surg Med 2006;38:881-9.[LinkOut]
- Freitag L, Ernst A, Thomas M, Prenzel R, Wahlers B, Macha HN. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax 2004;59:790-3.[LinkOut]
- Weinberg BD, Allison RR, Sibata C, Parent T, Downie G. Results of combined photodynamic therapy (PDT) and high dose rate brachytherapy (HDR) in treatment of obstructive endobronchial non-small cell lung cancer (NSCLC). Photodiagnosis Photodyn Ther 2010;7:50-8.[LinkOut]
- Sanfilippo NJ, Hsi A, DeNittis AS, Ginsberg GG, Kochman ML, Friedberg JS, et al. Toxicity of photodynamic therapy after combined external beam radiotherapy and intraluminal brachytherapy for carcinoma of the upper aerodigestive tract. Lasers Surg Med 2001;28:278-81.[LinkOut]
- Fujimura S, Sakurada A, Sagawa M, Saito Y, Takahashi H, Tanita T, et al. A therapeutic approach to roentgenographically occult squamous cell carcinoma of the lung. Cancer 2000;89:2445-8.[LinkOut]
- van Boxem TJ, Venmans BJ, Schramel FM, van Mourik JC, Golding RP, Postmus PE, et al. Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 1998;11:169-72.[LinkOut]
- Kubota K, Furuse K, Kawahara M, Kodama N, Yamamoto M, Ogawara M, [Photodynamic therapy of roentgenographically occult lung cancer]. Kyobu Geka 1992;45:80-3.[LinkOut]
- Ono R, Ikeda S, Suemasu K. Hematoporphyrin derivative photodynamic therapy in roentgenographically occult carcinoma of the tracheobronchial tree. Cancer 1992;69:1696-701.[LinkOut]
- Furuse K, Okunaka T, Sakai H, Konaka C, Kato H, Aoki M, et al. [Photodynamic therapy (PDT) in roentgenographically occult lung cancer by photofrin II and excimer dye laser]. Gan To Kagaku Ryoho 1993;20:1369-74.[LinkOut]
- Imamura S, Kusunoki Y, Takifuji N, Kudo S, Matsui K, Masuda N, et al. Photodynamic therapy and/or external beam radiation therapy for roentgenologically occult lung cancer. Cancer 1994;73:1608-14.[LinkOut]
- Endo C, Miyamoto A, Sakurada A, Aikawa H, Sagawa M, Sato M, et al. Results of long-term follow-up of photodynamic therapy for roentgenographically occult bronchogenic squamous cell carcinoma. Chest 2009;136:369-75.[LinkOut]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: a follow-up study. Lung Cancer 2003;39:49-53.[LinkOut]
- Cortese DA, Kinsey JH. Hematoporphyrin-derivative phototherapy for local treatment of cancer of the tracheobronchial tree. Ann Otol Rhinol Laryngol 1982;91:652-5.[LinkOut]
- Edell ES, Cortese DA. Bronchoscopic phototherapy with hematoporphyrin derivative for treatment of localized bronchogenic carcinoma: a 5-year experience. Mayo Clin Proc 1987;62:8-14.[LinkOut]
- Cortese DA, Edell ES, Kinsey JH. Photodynamic therapy for early stage squamous cell carcinoma of the lung. Mayo Clin Proc 1997;72:595-602.[LinkOut]
- Kato H, Okunaka T, Tsuchida T, Shibuya H, Fujino S, Ogawa K. Analysis of the Cost-effectiveness of Photodynamic Therapy in Early Stage Lung Cancer. Diagn Ther Endosc 1999;6:9-16.[LinkOut]
- Balchum OJ, Doiron DR, Huth GC. Photoradiation therapy of endobronchial lung cancers employing the photodynamic action of hematoporphyrin derivative. Lasers Surg Med 1984;4:13-30.[LinkOut]
- Li JH, Chen YP, Zhao SD, Zhang LT, Song SZ. Application of hematoporphyrin derivative and laser-induced photodynamical reaction in the treatment of lung cancer: a preliminary report on 21 cases. Lasers Surg Med 1984;4:31-7.[LinkOut]
- Liang YM, Tu QX, Liu GY, Zhang XS. [Short term result of lung cancer treated by photodynamic therapy (PDT)]. Zhonghua Zhong Liu Za Zhi 1987;9:50-2.[LinkOut]
- Furuse K, Fukuoka M, Kato H, Horai T, Kubota K, Kodama N, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol 1993;11:1852-7.[LinkOut]
- Kawaguchi T, Yamamoto S, Naka N, Okishio K, Atagi S, Ogawara M, et al. Immunohistochemical analysis of Bcl-2 protein in early squamous cell carcinoma of the bronchus treated with photodynamic therapy. Br J Cancer 2000;82:418-23.[LinkOut]
- McCaughan JS Jr, Williams TE. Photodynamic therapy for endobronchial malignant disease: a prospective fourteen-year study. J Thorac Cardiovasc Surg 1997;114:940-6; discussion 946-7.[LinkOut]
- Miyazu Y, Miyazawa T, Kurimoto N, Iwamoto Y, Kanoh K, Kohno N. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med 2002;165:832-7.[LinkOut]
- Patelli M, Lazzari Agli L, Poletti V, Falcone F. Photodynamic laser therapy for the treatment of early-stage bronchogenic carcinoma. Monaldi Arch Chest Dis 1999;54:315-8.[LinkOut]
- Ali AH, Takizawa H, Kondo K, Nakagawa Y, Toba H, Khasag N, et al. Follow-up using fluorescence bronchoscopy for the patients with photodynamic therapy treated early lung cancer. J Med Invest 2011;58:46-55.[LinkOut]
- Kato H, Okunaka T, Shimatani H. Photodynamic therapy for early stage bronchogenic carcinoma. J Clin Laser Med Surg 1996;14:235-8.[LinkOut]
- Kato H, Okunaka T, Konaka C, Furuse K, Kusunoki Y, Horai T, et al. Photodynamic Therapy With YAG-OPO Laser for Early Stage Lung Cancer. Diagn Ther Endosc 1997;4:75-81.[LinkOut]
- Furukawa K, Kato H, Konaka C, Okunaka T, Usuda J, Ebihara Y. Locally recurrent central-type early stage lung cancer LinkOut]
- Moghissi K, Dixon K. Update on the current indications, practice and results of photodynamic therapy (PDT) in early central lung cancer (ECLC). Photodiagnosis Photodyn Ther 2008;5:10-8.[LinkOut]
- Freitag L, Korupp A, Itzigehl I, Dankwart F, Tekolf E, Reichle G, et al. [Experiences with fluorescence diagnosis and photodynamic therapy in a multimodality therapy concept of operated, recurrent bronchial carcinoma]. Pneumologie 1996;50:693-9.[LinkOut]
- Moghissi K, Dixon K, Thorpe JA, Stringer M, Oxtoby C. Photodynamic therapy (PDT) in early central lung cancer: a treatment option for patients ineligible for surgical resection. Thorax 2007;62:391-5.[LinkOut]
- Corti L, Toniolo L, Boso C, Colaut F, Fiore D, Muzzio PC, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 2007;39:394-402.[LinkOut]
- Lam S. Photodynamic therapy of lung cancer. Semin Oncol 1994;21:15-9.[LinkOut]
- Okunaka T, Kato H, Tsutsui H, Ishizumi T, Ichinose S, Kuroiwa Y. Photodynamic therapy for peripheral lung cancer. Lung Cancer 2004;43:77-82.[LinkOut]
- Kato H, Furukawa K, Sato M, Okunaka T, Kusunoki Y, Kawahara M, et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003;42:103-11.[LinkOut]
- Usuda J, Ichinose S, Ishizumi T, Hayashi H, Ohtani K, Maehara S, et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin Cancer Res 2010;16:2198-204.[LinkOut]
- Chang YL, Wu CT, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg 2007;134:630-7.[LinkOut]
- Usuda J, Ichinose S, Ishizumi T, Hayashi H, Ohtani K, Maehara S, et al. Management of multiple primary lung cancer in patients with centrally located early cancer lesions. J Thorac Oncol 2010;5:62-8.[LinkOut]
- Rami-Porta R, Ball D, Crowley J, Giroux DJ, Jett J, Travis WD, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602.[LinkOut]
- Jung EJ, Lee JH, Jeon K, Koh WJ, Suh GY, Chung MP, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer 2011;73:237-42.[LinkOut]
- Saito M, Kato H, Konaka C, Okunaka T, Furukawa K, Sakai H, et al. Synchronous quadruple lung cancer treated curatively by photodynamic therapy. Diagn Ther Endosc 1996;3:115-9.[LinkOut]
- Okunaka T, Kato H, Konaka C, Kawate N, Bonaminio A, Yamamoto H, et al. Photodynamic therapy for multiple primary bronchogenic carcinoma. Cancer 1991;68:253-8.[LinkOut]
- Kodama K, Doi O, Tatsuta M, Higashiyama M, Iwanaga T. [Problems concerning the diagnosis and treatment of multiple primary carcinoma of the lung]. Kyobu Geka 1990;43:682-8.[LinkOut]
- Sokolov VV, Telegina LV, Trakhtenberg AKh, Kolbanov KI, Pikin OV, Frank GA. [Endobronchial surgery and photodynamic therapy for the treatment of multiple primary lung cancer]. Khirurgiia (Mosk) 2010;(7):28-31.[LinkOut]
Cite this article as: Simone CB 2nd, Friedberg JS, Glatstein E, Stevenson JP,
Sterman DH, Hahn SM, Cengel KA. Photodynamic therapy for the treatment
of non-small cell lung cancer. J Thorac Dis 2012;4(1):63-75. doi: 10.3978/
j.issn.2072-1439.2011.11.05
|