
Lymph node metastasis matters
The races to identify the specific populations that respond remarkably well or have dreadfully worst response to immune check point inhibitor (ICI) are hard-edged, especially because the cost of these drugs are so devastating that people are afraid that the widespread use will lead to the destruction of the insurance system and enhance the inequality of health among the patients according to individual economical status. The first naïve assumption was that PD-L1 expression in tumor cells would predict the response. The dynamics of PD-L1/PD-1 axis, however, was actually more complicated and unstable, thus the attributes other than those molecules themselves to predict the response continued to be searched for.
The most perplexing aspect of newly adopted ICIs, some of the patients look deteriorating after treatment. That situation cannot be accepted for patients, families, and attending doctors.
Before molecular analyses of this phenomenon are launched, the practical “facts” should be analyzed systematically. Non-small cell lung carcinoma is the most debated disease in terms of the options among several conventional options of treatment. The French group led by Ferrara et al. (1) reported a multi-institutional study on hyper-progressive disease (HPD) treated by PD-1/PD-L1 inhibitor and compared to the cohort treated by single-agent chemotherapy. HPD was defined in RECIST version 1.1 as a progressive disease on the first CT scan during treatment and ΔTGR (tumor growth rate) exceeding 50%, corresponding to an absolute increase in the TGR exceeding 50% per month.
The first question is whether HPD occurs more often in immune-oncology (IO) treated patients than those receiving conventional chemotherapy. The answer was yes. The percentage of HPD in a cohort was higher in ICI cohort than those treated with conventional chemotherapy (single arm). HPD, an unfamiliar category, had been proposed and defined in their previous paper (2). They draw attention of the oncologists in practice perplexed with the unconventional pattern of the response of ICI. These unconventional categories include pseudoprogression in addition to HPD, and documentation of them is accumulating in accordance with the increase in numbers of the cases given ICIs. The group including the authors of this paper even propose IRECIST as a new measurement system, the immune response was added to ordinary RECIST (3).
Going back to the original article, they found that 56/406 (13.8%) cases were defined as HPD. Further analysis disclosed HPD was associated with the presence of two and more metastatic sites at the beginning of ICI therapy. The study was retrospective multicentered study. And regimen of immuno-oncology (IO) therapy included nivolumab, pembrolizumab, atezolizumab, and durvalumab; both programmed cell death 1 (PD-1) and programme cell death ligand 1 (PD-L1) inhibitors. The data described here is basic clinical observation using conventional modalities, and the findings are instinctively persuasive.
But we should note the 6 of the 62 HPD cases (9.7%) obtained clinical response after the defined 6 weeks from the initiation of ICIs. There is a methodological problem; the definition of HPD such as a growth rate and duration depends on the study design (4-6), and it may possibly include the pseudo-progressive cases mistakenly. Pseudo-progression is a tricky concept emerging since ICIs began to be used (7), which also require further investigations to clarify the concept and pathogenesis. Thus in the specific cases, the clinical response of ICIs might need to be carefully judged from a relatively long-term perspective compared with the patients who received conventional treatments.
Also it is also an important issue to consider genetic abnormality of HPD existing in the background. In this study, they did not extend their study to comprehensive genetic analysis. Recently, Kato and colleagues reported that MDM2 family amplification and EGFR alterations are clinically relevant to HPD (8) but the cases with HPD in their study was only four cases; sample number is quite small. A thorough investigation of the genetic relationship with HPD is still a challenge in this field and the genetic abnormalities underlying HPD might be revealed in the era of clinical sequencing by next generation sequencing.
There are many potential attributes to predict the effectiveness of ICI. One of the areas is the characterization of PD-L1 role in tumor cells. Semi-quantitative estimation of immunohistochemical expression in tumor cells is proposed to predict the effectiveness in several ICI, not in the others.
Robust immunohistochemical predictors are still challenges for many pathologists attending the cancer immunotherapy (9,10). Another way of estimation of PD-L1 in tumor cells is genomic ones ranging from amplification (11,12) to structural changes in the non-coding region (13,14) using NGS. Tumor mutation burden itself is also thought to be a significant predictor of the effectiveness of IO, thus exome analysis or mismatch repair deficiency assessment in the tumor cells could be essential for practice in the future. Other important area is tumor microenvironment. Tumor infiltrating lymphocytes including CD8 subset of T cells and PD1 positive lymphocytes (Figure 1) are believed to be protective. In addition to this easily countable index, the metabolic index including metabolic mediator of immune escape such as IDO pathway (15) and microbiome (16).
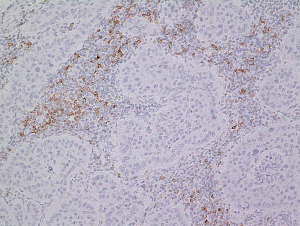
Then what happened in metastatic lymph nodes? The recent presentation, demonstrated spatiotemporal heterogeneity of PD-L1 immunohistochemical expressions and copy number variations, highlighting the importance of understanding of progressed tumors as a complexed biological situation (17). The observations like that by Ferrara et al. (1) will continue to draw our attention to the whole tumors in the progressed stage including metastatic niche.
Acknowledgements
Funding: This work was funded by AMED (17ck0106264h0001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4:1543-52. [Crossref] [PubMed]
- Ferrara R, Pilotto S, Caccese M, et al. Do immune checkpoint inhibitors need new studies methodology? J Thorac Dis 2018;10:S1564-S1580. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-e152. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Gandara DR, Von Pawel J, Sullivan RN. Impact of atezolizumab(atezo) treatment beyond disease progression (TBP) in advanced NSCLC: results from the randomized phase III OAK study. J Clin Oncol 2017;35:abstr 9001.
- Kato S, Ross JS, Gay L, et al. Analysis of MDM2 Amplification: Next-Generation Sequencing of Patients With Diverse Malignancies. JCO Precis Oncol 2018;2018.
- Cree IA, Booton R, Cane P, et al. PD-L1 testing for lung cancer in the UK: recognizing the challenges for implementation. Histopathology 2016;69:177-86. [Crossref] [PubMed]
- Cagle PT, Bernicker EH. Challenges to Biomarker Testing for PD-1/PD-L1 Checkpoint Inhibitors for Lung Cancer. Arch Pathol Lab Med 2015;139:1477-8. [Crossref] [PubMed]
- Ikeda S, Okamoto T, Okano S, et al. PD-L1 Is Upregulated by Simultaneous Amplification of the PD-L1 and JAK2 Genes in Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:62-71. [Crossref] [PubMed]
- Inoue Y, Yoshimura K, Mori K, et al. Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget 2016;7:32113-28. [Crossref] [PubMed]
- Kataoka K, Ogawa S. Genetic biomarkers for PD-1/PD-L1 blockade therapy. Oncoscience 2016;3:311-2. [PubMed]
- Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD-L1 expression through 3'-UTR disruption in multiple cancers. Nature 2016;534:402-6. [Crossref] [PubMed]
- Muller AJ, Manfredi MG, Zakharia Y, et al. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol 2019;41:41-48. [PubMed]
- Oliva M, Spreafico A, Taberna M, et al. Immune Biomarkers of Response to Immune-Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma. Ann Oncol 2019;30:57-67. [Crossref] [PubMed]
- Yoshimura K, Inoue Y, Tsuchiya K, et al. Heterogeneity Analysis of EBUS-TBNA-Derived Specimens for Evaluation of PD-L1 Expression and Copy Number Alterations in Patients with NSCLC. J Thoracic Oncol 2018;13:S400-1. [Crossref]


