
Perspective on airway stenting in inoperable patients with tracheoesophageal fistula after curative-intent treatment for esophageal cancer
Definitions, prevalence, anatomical considerations and risk factors
The term esophago-respiratory fistula, aero-digestive and tracheoesophageal fistula (TEF) have been used to describe the abnormal connection of the airways with the digestive system as this connection can be between the bronchus or the trachea and the esophagus or stomach. In patients who are post esophagectomy, aero-gastric fistula (usually tracheo-gastric) can develop between the gastric pull up and the airway (trachea or main stem bronchi). Histologic classification of TEF post treatment for esophageal cancer is important as treatment for malignant TEF is usually palliative and very rarely involves surgical correction. Malignant TEF occurs secondary to invasion of a locally-advanced esophageal, tracheal or lung cancer (1). In one large case series, the majority of malignant TEFs were due to esophageal cancer (92% of TEF cases) with lung cancer being responsible for 7% and mediastinal tumors for 1% (2,3). TEF is seen in 5–15% of patients with esophageal cancer (1-5). Histologically benign TEFs usually result from complications of indwelling tracheal or esophageal stents, esophageal or tracheal surgery, granulomatous mediastinal infection (tuberculosis, histoplasmosis), trauma (penetrating or blunt) or ingestion or aspiration of caustics or foreign bodies (6).
The trachea and the esophagus originate from the embryonic foregut and remain juxtaposed in the superior mediastinum. The trachea and proximal left main stem bronchus are anterior to the esophagus. Thus, tumors originating from the esophagus can invade into the thin membranous posterior tracheal wall. This anatomical proximity to the airway, advanced cancer stage, and upper-mid esophageal tumors are risk factors for the development of a TEF (3). TEF also often occurs as a consequence of surgical interventions or chemoradiotherapy treatment for esophageal cancers. When no residual tumor is present, TEFs should be considered and managed as benign entities.
Chemoradiotherapy is offered in several circumstances in the management of esophageal cancer, especially once tumors extend into the trachea (7). Tumor necrosis due to radiation therapy (most common) or chemotherapy (especially with antiangiogenesis agents) increase the risk for TEF development (1,8-10). In a large retrospective study, radiation was reported as a primary treatment in 65–70% of patients diagnosed with TEF, after a median time of 347 days (1,3). In contrast, in another study, 15.5% of the 264 patients had radiation treatment with only 10% of the TEF considered to be ‘irradiation-induced’ (defined as occurring within 4 weeks) (2).
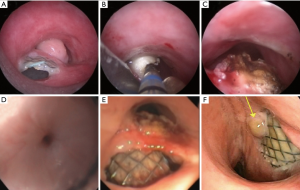
Esophageal stents inserted for strictures may also cause pressure necrosis and consequent TEF formation at the level of treated tumor or at the ends of the stent (Figure 1) (10). While reportedly this is an uncommon complication of esophageal stenting (~4%), in one study the risk was increased in patients who received prior radiation and had a higher Charlson comorbidity index score (11). From retrospective data, however, it is often difficult to determine whether a TEF after curative-intent treatment for esophageal cancer is directly caused by the treatment or a consequence of cancer-progression/recurrence seen in longer surviving patients (2).
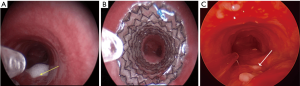
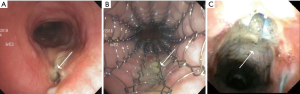
Esophagectomy with gastric pull is the most reliable curative intervention for early-stage esophageal cancer. A tracheo-gastric fistula (TGF) complicates this surgery in 0.3–1.9% of these cases and presents a unique management challenge, as re-operation or gastric stenting may not be feasible (Figure 2) (12-14). TGF can occur in the early post-operative period secondary to mediastinitis or tracheal injury, or late, secondary to erosion, tumor recurrence or radiation. Risk factors for the development of TGF include perioperative radiation, peritracheal lymph node dissection, and ischemia associated with resection of bronchial and inferior thyroid arteries (15). In one study, all patients with a TGF had a metachronous or synchronous anastomotic leak of the esophagogastrostomy (12).
Treatment modalities
The goal of treatment in patients with TEF is to prevent aspiration and consequent pneumonia, and to improve nutritional status. Supportive care including nutrition through a gastrostomy or jejunostomy tube or parenterally, intravenous hydration, and antibiotics is offered to most patients. Patients with TEF have survival measured in weeks with supportive care alone (1,3,16). Some patients are or could become candidates for corrective surgery once performance status, nutrition and aspiration have improved. Collar esophagostomy with gastrostomy or jejunostomy for nutrition, bypass with anatomical reconstruction of the gastrointestinal tract, or primary fistula closure using healthy tissue (e.g., muscle) with diversion of the source of insult (e.g., using a nasogastric tube) are surgical treatments that have been performed for this pathology (4). However, major surgery is often not feasible or is associated with high complication (up to 40%) and mortality (up to 14%) rates in this usually debilitated and malnourished population (1,17-19). Surgical treatment is thus reserved for large fistulas in patients who can tolerate major operations (20). As the overall health and performance status of these patients is usually prohibitive to surgery, interventional bronchoscopic or endoscopic stenting, with the goal of covering the fistulous communication, is the palliative procedure of choice in most patients with TEF after esophageal cancer treatment. In fact, published literature suggests that stenting can prolong survival by several months (1,2,16,21).
Esophageal stenting
The goal of esophageal stenting is to allow oral feeding and prevent respiratory tract contamination. Stenting the esophagus is the logical first step in patients with a TEF with or without an esophageal stricture, as it acts as a conduit for natural downward movement of oral secretions. The esophageal wall perfectly molds around the esophageal stent thus assuring an intimate contact between the stent and the mucosa which will prevent spillage of secretions in the airways (4). The length of the esophagus allows operators to select a longer stent to safely extend proximal and distal to the fistula (i.e., at least 2 cm on either end) (Figure 1) (4).
Esophageal self-expanding metal stents (SEMS) have become the prosthesis of choice for TEF, replacing the previously used plastic prostheses due to their lower migration rate and the provision of a better seal (3,5,19,21). Esophageal stenting has been shown to improve quality of life and to effectively palliate aspiration symptoms and prolong survival (when compared to airway stenting) (21).
The most common esophageal location of a TEF is in the middle third of the esophagus (2,3,22). Technical difficulty in placing esophageal stents is encountered when endoscopic access to the TEF is challenged by a proximal stricture, or the TEF is located high in the cervical esophagus making stenting difficult (due to the upper esophageal sphincter) or risky (due to the risk of airway compromise at this level where the mediastinum is narrower). In one study, the authors reviewed 39 patients who had esophageal stents inserted for a TEF, and noted that 10 (25%) required subsequent silicone airway stent placement due to airway compression (19). Esophageal stents do not necessarily prevent aspiration, especially when placed in the distal third of the esophagus (as they may worsen reflux). The peri-procedure complication rates and stent-related mortality of esophageal stenting is 0–17% and 0–2%, respectively (2,16,23). In addition, they could compress or further erode into the airway, complicating the management of this already challenging condition.
Airway stenting
On the airway side, the location of TEF is more common in the trachea than in either main stem bronchi (1,3). Distal esophageal fistulas are more likely to communicate with major bronchi. The goal of airway stenting is to cover the fistula by maintaining adequate contact with the airway wall (by appropriate sizing and stent radial expansile force) to not only minimize spillage of oral secretion into the respiratory tract, but also to prevent stent migration. The non-circular airway shape, presence of airway cartilage, lack of airway obstruction (in many cases with TEF post esophagectomy or post chemoradiation) and dynamic respiration changes of airways make complete wall approximation and TEF-covering difficult with airway stents. These facts, in addition to the potentially more severe complications caused by airway stenting compared to esophageal stenting (e.g., airway obstruction caused by granulation tissue, mucostasis, migration, airway perforation) make airway stents a less appealing first choice intervention for TEF (Figure 3). Besides, airway stents often require surveillance bronchoscopy and frequent saline nebulization for humidification, a practice not applicable for esophageal stenting (4).
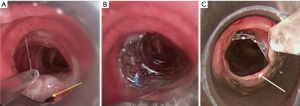
Airway stenting should be considered prior to esophageal stenting only when: (I) there is pre-existing symptomatic airway obstruction; (II) symptomatic airway obstruction develops post esophageal stent insertion (and the esophageal stent could not be replaced); (III) the esophageal stent did not successfully cover the fistula or (IV) the esophageal stent could not be inserted (due to a stricture that causes difficulty passing the scope or the guidewire distal to the TEF—usually due to a proximal esophageal location, or due to extensive esophageal necrosis prohibiting safe placement) (4,9,21). If the airway stent is inserted first (such as in the case of fistula with concurrent airway obstruction), subsequent esophageal stenting should be considered if there is incomplete fistula approximation as evidenced endoscopically or with a contrast dye leak test (9,21,24).
In the situation of a TGF, if there is an anastomotic leak or gastric necrosis, re-thoracotomy should be pursued (12). In other patients, especially if the operative risk is prohibitive, SEMS can be placed in the airway or less commonly in the gastric pull up. Stenting the airway first is often considered, as stenting the newly created conduit is not always feasible (Figure 2). The airway site of the fistula is usually the trachea or the left main bronchus. Airway stenting for TGF has an initial success of 75%, but is associated with a high fistula recurrence rate (39%), and thus must be viewed as a temporizing step until the patient can be considered appropriate for surgery (13,14). In some cases, the TGF can completely heal once the stent has been in place for several weeks, in which case surgery is of course not indicated or necessary (Figure 2). Limited published literature suggests that durable TGF and broncho-gastric fistula closure with airway stents can be achieved in 12.5% and 60% cases, respectively (13). Covered SEMS were used in most patients (75%). Only 9% of the patients surviving more than 3 months had to undergo definitive surgery.
Choice of stent
The choice of airway stents is dependent on various factors including the presence or absence of airway obstruction as well as extent and location of the fistula (Figures 4-6). Airway stent selection is challenging due to the airway branching points (main, primary or secondary carinas), the variability in airway diameters (trachea, right and left main bronchi), thickness and adjacent structures of the different airways. Radial force refers to the force that the stent employs on the airway wall. This is higher for stents made of silastic than with SEMS (25). When a fistula is fully covered by the stent but there is significant tension on the already disrupted airway wall, the fistula could enlarge in the presence of indwelling stents with high radial (i.e., expansile) force.” In addition, their lack of resistance to buckling and stiffness may a prevent perfect configuration to the airway walls, especially when no airway compression/obstruction is present. On the other hand, silicone stents (i.e., Dumon type) have studs on their outer wall which may prevent migration that is commonly seen with fully covered SEMS. Besides, even when silicone stents are indicated, they can only be inserted using rigid bronchoscopy. In a patient with limited cervical spine extension or mouth opening or in a facility where expertise in rigid bronchoscopy is unavailable, flexible bronchoscopic insertion of covered SEMS or partially covered SEMS are reasonable choices. Review of the literature suggests that when compared to silicone stents, SEMS results in improved secretion clearance, more precise accommodation to tracheal dimensions, and lower incidence of migration in the management of TEF (26). In a study of 63 patients with malignant TEF, complete closure was achieved in 45 patients (71%) (27). If a partially covered SEMS is selected, the operators need to assure that the TEF is fully covered by the covered portion of the stent. Silicone, and some SEMS stents are available in straight and Y-shapes allowing for use in carinal or proximal main stem bronchial fistulas. Both silicone and hybrid Y stents have been successfully used for TEF. The largest trial with the hybrid Dynamic Y stent included 135 patients with central airway stenosis (malignant or benign) and TEFs. At three months follow up, two patients died of hemoptysis secondary to erosion into vascular structure and in four patients there was cephalad migration of the stent (28). Placement of Y stents in general could be technically challenging and poses the risk for enlargement of the fistula, and should be performed by teams versed in the Y stent insertion techniques (Figure 4).
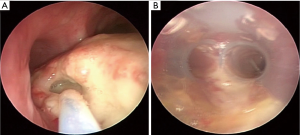
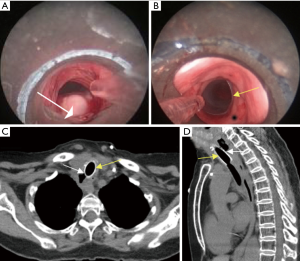
Covered or partially covered SEMS allow better contact with the airway wall. The “shape memory” of the nitinol alloy used in these stents and the covering (e.g., polyurethane or silicone) allows for complete TEF occlusion (4). Stents with uncovered ends (i.e., partially covered SEMS; e.g., UltraflexTM, Boston Scientific, Natick, MA, USA) may have lower migration rates, but the operators need to assure the TEF is fully covered by the covered portion of the stent (Figure 5). Using stents with flared ends, as is often used in the esophagus to mitigate migration, may not permit optimal wall approximation required to completely cover a TEF in the less pliable (cartilaginous) airways, especially in the absence of airway compression (Figure 3). SEMS can be inserted under fluoroscopic guidance or under direct visualization (4). We prefer direct visualization either through a rigid bronchoscope or by inserting the flexible bronchoscope next to the endotracheal tube (ETT). Even regular bronchoscopes with outer diameters of 4.2–5.5 mm can be used for this purpose. In this technique, the guidewire is advanced through the bronchoscope while it is in the ETT (usually size 7–8) and above the obstruction/fistula. Then the scope is removed, leaving the guidewire in place. The scope is re-inserted along the ETT and advanced into the space between the ETT and tracheal wall. This is possible in most patients after deflating the ETT cuff, but depends on the size of the ETT and patient’s tracheal diameter. The stent delivery catheter is then inserted over the guidewire and the SEMS is deployed under direct bronchoscopic visualization (29).
Straight stents (silicone or hybrid) should be long enough to extend 2 cm beyond each end of the fistula (if possible), and sized to be 10–20% larger than the airway diameter (4). The usual “rule of thumb” for airway stenting (i.e., to overlap the airway abnormality by 2–1 cm proximally, 1 cm distally), may not apply for TEF post chemoradiotherapy/post esophagectomy, especially if no tumor is present to compress the stent. A short overlapping segment will result in either residual leak or migration (Figure 3). Undersizing will also lead to migration, while oversizing can impair mucosal blood flow, interfering with healing and potentially extending the TEF. Granulation tissue at the stent ends is often caused by stent oversizing, and should warrant scheduled surveillance bronchoscopies (30). We routinely perform surveillance bronchoscopy at 4–6 weeks post stent insertion. Subsequent follow up procedures depend on bronchoscopic findings and overall goals of treatment. Especially for patients with TEF, bronchoscopy can determine whether fistula has healed or progressed, or whether the stent has migrated (scenarios that will all require stent revision or removal). Chest computed tomography (CT) may be used to follow-up airway stents for a variety of indications (Figure 6) (31). CT is relatively accurate in demonstrating the location and extent of endobronchial abnormalities, but is limited in demonstrating subtle narrowing (32). Three dimensional volume-rendered images, multiplanar reconstructions and minimum intensity projection images reconstructed along the airway axis may be very helpful to detect airway pathology and stent-related complications (33,34). CT can also serve as guide for planning subsequent bronchoscopic interventions.
Double stenting
No studies to date were designed to detect a difference in efficacy or safety between double stenting and standalone esophageal or airway stenting. The evidence available on this technique is based on case series (19,21,24). Most patients with esophageal cancer treated with chemoradiotherapy who develop TEF and are inoperable will be successfully palliated through an esophageal stent alone, with double stenting indicated in only specific circumstances as outlined above. In a prospective study of 112 patients with TEF, double stenting was required in 9 percent (21). Results of case series suggest that double stenting may improve survival in patients with malignant TEF compared to airway-alone stenting (21,24). In another study, 96% of patient who underwent double stenting achieved complete “response” (defined as no leak of contrast dye on digital radiography, and resolution of symptoms without recurrence for more than 2 weeks) compared to only 67% of those who underwent airway stenting (9).
Some experts suggest that double stenting should be considered when there is a large fistula (>2 cm) that may not be adequately covered by an esophageal stent alone, or when airway compromise is caused by extrinsic compression after esophageal stent expansion. Four (40%) of out the 10 patients in a study by Colt et al., who required double stenting, did so due to airway comprise caused by an esophageal stent (19). In our opinion, this latter scenario (in which there is a fistula and compression from an existing esophageal stent) is not uncommon and ideally the esophageal stent should be revised. If that is not possible or if there is persistent fistula despite esophageal stent revision, then an airway stent can be inserted, understanding that the TEF may enlarge with time and further surgical interventions may never be possible (21,35). Therefore, clinical suspicion for this potential scenario of airway compromise after esophageal stenting would justify an airway inspection bronchoscopy prior to and immediately after esophageal stenting. Double stenting would thus be considered when there is pre-stenting airway obstruction (wherein the airway stent should be placed first and an esophageal stent may be placed thereafter, if need be for dysphagia or persistent fistula) (9). Airway stents can also be placed to supplement an esophageal stent that doesn’t adequately cover the TEF (i.e., when a subsequent contrast dye study shows leak into the respiratory tract despite the esophageal stent) (21). We warn that such an approach will commit patients to a high risk for TEF enlargement. It would be a flagrant misconception to believe that the fistula will heal when there is pressure from two foreign objects (i.e., airway and esophageal stents) on both sides of the airway membranous wall. Therefore, in patients with TEF who have successfully seen curative treatment, who may go on to become surgical candidates or survive long-term, double stenting should be avoided as the friction between stents may compromise the healing process.
Summary and recommendations (Figure 7)
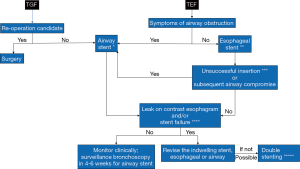
TEF is not an uncommon complication of curative-intent treatment for esophageal cancers, but its exact incidence is difficult to determine from the available, predominantly retrospective, case series. Surgical corrective options are not always feasible due to poor performance, necrosis at the fistula site and nutritional status of these patients. In situations when feasible, esophageal stenting alone should be initially pursued to cover the fistula to prevent spillage of oral or gastric secretions into the respiratory tract. Only when the results are not satisfactory after esophageal stenting (incomplete endoscopic or radiographic TEF-covering) or when the esophageal stent causes airway compression, should airway stenting (i.e., airway alone or double stenting) be considered. Airway stents should be placed first (i.e., prior to the esophageal stent) when there is clinically evident pre-existing airway obstruction, or asymptomatic obstruction expected to worsen after esophageal stenting. Otherwise, due to imperfect approximation with the airway wall and their potentially severe complications, airway stents should not be routinely used as the initial intervention. Follow up bronchoscopy is warranted at 4–6 weeks post airway stent insertion to determine whether the stent is still necessary or whether complications have occurred, which may require further management.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Burt M, Diehl W, Martini N, et al. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg 1991;52:1222-8. [Crossref] [PubMed]
- Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg 2008;34:1103-7. [Crossref] [PubMed]
- Choi MK, Park YH, Hong JY, et al. Clinical implications of esophagorespiratory fistulae in patients with esophageal squamous cell carcinoma (SCCA). Med Oncol 2010;27:1234-8. [Crossref] [PubMed]
- Hürtgen M, Herber SCA. Treatment of malignant tracheoesophageal fistula. Thorac Surg Clin 2014;24:117-27. [Crossref] [PubMed]
- Spaander MC, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:939-48. [Crossref] [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Ke M, Wu X, Zeng J. The treatment strategy for tracheoesophageal fistula. J Thorac Dis 2015;7:S389-97. [PubMed]
- Shamji FM, Inculet R. Management of Malignant Tracheoesophageal Fistula. Thorac Surg Clin 2018;28:393-402. [Crossref] [PubMed]
- Bick BL, Song LM, Buttar NS, et al. Stent-associated esophagorespiratory fistulas: incidence and risk factors. Gastrointest Endosc 2013;77:181-9. [Crossref] [PubMed]
- Lambertz R, Hölscher AH, Bludau M, et al. Management of Tracheo- or Bronchoesophageal Fistula After Ivor-Lewis Esophagectomy. World J Surg 2016;40:1680-7. [Crossref] [PubMed]
- Boyd M, Rubio E. The utility of stenting in the treatment of airway gastric fistula after esophagectomy for esophageal cancer. J Bronchology Interv Pulmonol 2012;19:232-6. [Crossref] [PubMed]
- Sahebazamani M, Rubio E, Boyd M. Airway gastric fistula after esophagectomy for esophageal cancer. Ann Thorac Surg 2012;93:988-90. [Crossref] [PubMed]
- Yasuda T, Sugimura K, Yamasaki M, et al. Ten cases of gastro-tracheobronchial fistula: a serious complication after esophagectomy and reconstruction using posterior mediastinal gastric tube. Dis Esophagus 2012;25:687-93. [Crossref] [PubMed]
- Chen YH, Li SH, Chiu YC, et al. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One 2012;7:e42766. [Crossref] [PubMed]
- Davydov M, Stilidi I, Bokhyan V, et al. Surgical treatment of esophageal carcinoma complicated by fistulas. Eur J Cardiothorac Surg 2001;20:405-8. [Crossref] [PubMed]
- Duranceau A, Jamieson GG. Malignant tracheoesophageal fistula. Ann Thorac Surg 1984;37:346-54. [Crossref] [PubMed]
- Colt HG, Meric B, Dumon JF. Double stents for carcinoma of the esophagus invading the tracheo-bronchial tree. Gastrointest Endosc 1992;38:485-9. [Crossref] [PubMed]
- Lenz CJ, Bick BL, Katzka D, et al. Esophagorespiratory Fistulas: Survival and Outcomes of Treatment. J Clin Gastroenterol 2018;52:131-6. [PubMed]
- Herth FJ, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010;36:1370-4. [Crossref] [PubMed]
- Raijman I, Siddique I, Ajani J, et al. Palliation of malignant dysphagia and fistulae with coated expandable metal stents: experience with 101 patients. Gastrointest Endosc 1998;48:172-9. [Crossref] [PubMed]
- Sarper A, Oz N, Cihangir C, et al. The efficacy of self-expanding metal stents for palliation of malignant esophageal strictures and fistulas. Eur J Cardiothorac Surg 2003;23:794-8. [Crossref] [PubMed]
- Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest 1996;110:1155-60. [Crossref] [PubMed]
- Freitag L. Airway stents. In: Strausz J, Bolliger CT. Interventional Pulmonology. European Respiratory Society. 2010.
- Zhou C, Hu Y, Xiao Y, et al. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis 2017;11:173-80. [Crossref] [PubMed]
- Wang H, Tao M, Zhang N, et al. Airway Covered Metallic Stent Based on Different Fistula Location and Size in Malignant Tracheoesophageal Fistula. Am J Med Sci 2015;350:364-8. [Crossref] [PubMed]
- Freitag L, Tekolf E, Stamatis G, et al. Clinical evaluation of a new bifurcated dynamic airway stent: a 5-year experience with 135 patients. Thorac Cardiovasc Surg 1997;45:6-12. [Crossref] [PubMed]
- Wilson GE, Walshaw MJ, Hind CR. Treatment of large airway obstruction in lung cancer using expandable metal stents inserted under direct vision via the fibreoptic bronchoscope. Thorax 1996;51:248-52. [Crossref] [PubMed]
- Lee HJ, Labaki W, Yu DH, et al. Airway stent complications: the role of follow-up bronchoscopy as a surveillance method. J Thorac Dis 2017;9:4651-9. [Crossref] [PubMed]
- Ferretti GR, Kocier M, Calaque O, et al. Follow-up after stent insertion in the tracheobronchial tree: role of helical computed tomography in comparison with fiberoptic bronchoscopy. Eur Radiol 2003;13:1172-8. [PubMed]
- Ferretti GR, Knoplioch J, Bricault I, et al. Central airway stenoses: preliminary results of spiral-CT-generated virtual bronchoscopy simulations in 29 patients. Eur Radiol 1997;7:854-9. [Crossref] [PubMed]
- Dialani V, Ernst A, Sun M, et al. MDCT detection of airway stent complications: comparison with bronchoscopy. AJR Am J Roentgenol 2008;191:1576-80. [Crossref] [PubMed]
- Godoy MC, Saldana DA, Rao PP, et al. Multidetector CT evaluation of airway stents: what the radiologist should know. Radiographics 2014;34:1793-806. [Crossref] [PubMed]
- Nomori H, Horio H, Imazu Y, et al. Double stenting for esophageal and tracheobronchial stenoses. Ann Thorac Surg 2000;70:1803-7. [Crossref] [PubMed]


