The SNPs (-1654C/T, -1641A/G and -1476A/T) of protein C promoter are associated with susceptibility to pulmonary thromboembolism in a Chinese population
Introduction
Pulmonary thromboembolism (PTE) is a common and potentially lethal disease. There are the abnormal alterations in blood flow and the properties of the blood and the vessel wall associated with PTE. In recent years, the studies have revealed that thromboembolism was associated with single nucleotide polymorphism (SNP) and some gene mutations were attributable to PTE (1-12). Several distinct mutations in the 5'-promoter region of protein C gene have been identified (12). Theoretically, the gene mutations in the promoter may cause the express down-regulation of protein C and break the balance of the anticoagulation system and increase the risk of PTE. Therefore, it is significant to investigate the gene mutations of protein C for clarifying the etiology of PTE. In this present study, we investigated the promoter region polymorphism sites including -1654C/T, -1641A/G and -1476A/T of the protein C gene to explore their relationship with PTE.
Materials and methods
Participants
PTE group included one hundred ten patients continuously recruited from Department of Respiratory Medicine Shanghai Pulmonary Hospital from June 2003 to June 2010. All the patients were definitely diagnosed as PTE by radionuclide pulmonary perfusion imaging and CTPA or pulmonary angiography. Pulmonary angiography was assessed according to the Guidelines on Diagnosis and Management of PTE by Respiratory Society of Chinese Medical Association [2001]. The diagnosis of deep vein thrombosis (DVT) of lower limb vessels were performed by radionuclide venography and (or) ultrasonic examination. In this study, 190 healthy subjects from a health care center were recruited as the controls. None of the controls had a history of arterial disease (stroke, myocardial infarction, angina, or peripheral vascular disease), venous thrombosis (PTE or DVT), or functional lesion of the liver and kidney. There was no difference in age and sex between two groups (P<0.05).
Ethics statement
All subjects were treated in accordance with the Declaration of Helsinki on the participation of human subjects in medical research. Written informed consent was obtained from each of participants and the study was approved by the Ethics Committee of Shanghai Pulmonary Hospital.
Blood sampling
Venous blood samples from the patients and the controls were collected in 0.129 M trisodium citrate (1:9) tubes. After centrifugation, plasma was aliquoted and stored at –20 °C. A double-blind method was conducted to avoid the bias of tests.
PCR amplification and production purification
DNA was extracted by a commercial DNA extraction kit (Chaoshi Biotechnology, shanghai, China). Primer sequences were designed by the Oligo 6 software based on the DNA sequences of the protein C gene in GenBank (http://www.ncbi.nlm.nih.gov). The pair of primer sequences was listed as follows: 5'-ATTGGGATGGCATGTCATTG-3' and 5'-CCCTGGCTGGAGGATTCAG-3'. The expected PCR amplification included the three SNP sites (-1654C/T, -1641A/G and -1476A/T).
PCR amplifications were performed in a 50 µL reaction mixture containing 5 µL 10× PCR amplification buffer (100 mm of Tris-HCl, pH 8.3, 500 mm of KCl, 15 mm of MgCl2), 4 µL dNTP (2.5 mm of each), 20 pM of each primers, 2.5 unit of Taq polymerase and 200 ng genomic DNA. The PCR protocol is as following: step 1, 95 °C for 5 m; step 2, (95 °C for 45 s, 58 °C for 45 s, and 72 °C for 60 s) 35 cycles; step 3, 72 °C for 7 m. The PCR products were verified by electrophoresis on a 1% agarose gel with ethidium bromide staining. Finally, PCR reaction products were purified by using QIA PCR purification kit (Qiagen, Hilden, Germay).
DNA sequencing
These samples were further analyzed by direct DNA sequencing to identify the polymorphisms. The sequencing reactions were done on double-strand DNA with the above primers used in the PCR. The sequencing reactions were performed by the ABI PRISM 3730 DNA analyzer (Applied Biosystems, Foster City, USA).
Statistical analysis
Categorical variables were presented using frequency counts. Chi-square test is given as 2-sided Pearson’s Chi-Square and Fisher’s exact test was used to distinguish the differences in the genotype/allele/haplotype frequencies of the SNPs. Hardy–Weinberg equilibrium was used to verify allele frequency and genotype distribution. Binary logistic regression analysis was performed to evaluate the odds ratios of PTE. The data analysis used Stata 11.0 software (Statacorp LP, Texas, USA).
Results
Characteristics of participants
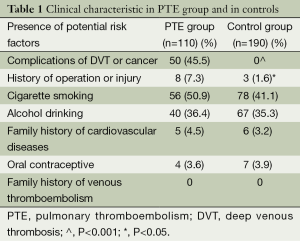
General characteristics of the study are shown in Table 1. There was significantly difference in the indices involving history of operation or injury, and the complications from DVT or cancer between the PTE group and the controls (P<0.05). No significant difference was found in these indices including family history of venous thromboembolism and cardiovascular diseases, personal history of oral contraceptive, cigarette smoking and alcohol drinking between the two groups (P>0.05).
Full table
Genotype and allele frequencies of three SNPs of the protein C gene
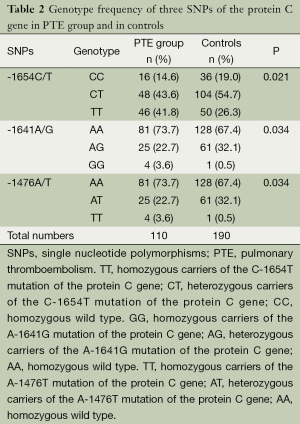
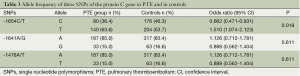
The SNP site (-1654C/T) was divided into CC, CT and TT genotype, while there were AA, AG, and GG genotype in -1641A/G and AA, AT, TT genotype in -1476A/T. Frequencies of genotype/allele between the two groups are described in Tables 2,3. Both the genotype frequencies of the three SNP sites and the allele frequencies of the SNP site (-1654C/T) were significantly different between two groups (P<0.05).
Full table
Full table
Distribution of the poly-TAA/CAA/CGT genotypes and haplotype frequencies of three SNPs of the protein C gene
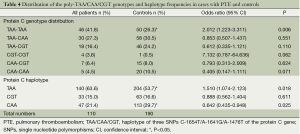
There are six kinds of genotypes (TAA-TAA, TAA-CAA, TAA-CGT, CGT-CGT, CAA-CGT, CAA-CAA) and three kinds of haplotypes (TAA, CGT, CAA), shown in Table 4. The frequency of haplotype TAA, especially the homozygous carriers with haplotype TAA (TAA-TAA) in the PTE group was significantly higher (P<0.05). On the contrary, the frequency of haplotype CAA in the PTE group was significantly lower than that in the controls (P<0.05). There was no difference in haplotype CGT between the two groups (P>0.05).
Full table
Risk factors for PTE by logistic regression
Binary logistic regression analysis showed that the independently significant risk factors for PTE were the complications of DVT or cancer, history of operation or injury, and the homozygous carriers of the TT genotype in -1654C/T (P<0.05). However, the factors including oral contraceptive, cigarette smoking, and alcohol drinking weren’t the significant risk factors for PTE (P>0.05).
Discussion
PTE is a common clinical problem which is associated with substantial morbidity and mortality (13). The incidence of PTE in the United States is estimated to be one case per 1,000 persons per year (13). The prospective investigation of pulmonary thromboembolism diagnosis (PIOPED) study showed that the 1-year mortality rate was 24% (14).
The risk factors of PTE are complex. However, accumulated evidences have shown that protein C plays an important role in PTE (9,11-25). Protein C is an important regulator of thrombin activity and the deficiency of protein C can result in the down-regulation of the coagulation system by inactivating factor Va and factor VIIIa. There are several transcriptional regulatory regions in the 5'-flanking sequence of the protein C gene, which regulates the expression of protein C. Some distinct mutations situated at the 5'-promoter region of human protein C gene have been identified (12). The polymorphic sites (-1654C/T, -1641A/G and -1476A/T) of the protein C gene have been shown to correlate with the incidence of DVT in Western countries (9,11).
In this study, we investigated the three SNPs in the protein C gene in PTE in a Chinese population. We found that both the genotype frequencies of three SNP sites (-1654C/T, -1641A/G and -1476A/T) and the C and T allele frequencies of -1654C/T were significantly different between PTE group and control group. The findings revealed that TT genotype of -1654C/T, GG genotype of -1641A/G and TT genotype of -1476A/T are significantly associated with the susceptibility to PTE in a Chinese population. The further sub-analysis showed that only TT genotype of -1654C/T was a significant risk factor for PTE (OR 2.245, 95% CI, 1.252 to 4.027), while neither GG genotype of -1641A/G nor TT genotype of -1476A/T was.
In this present study, we found that there were six kinds of genotype distribution (TAA-TAA, TAA-CAA, TAA-CGT, CGT-CGT, CAA-CGT, CAA-CAA) and three kinds of haplotype (TAA, CGT, CAA) in the included subjects. The frequency of TAA haplotype, particularly the homozygous carriers of TAA haplotype (TAA-TAA), was higher, while the frequency of CAA haplotype was lower in PTE. We found that allele T of -1654C/T is a risk factor to PTE and that allele C of -1654C/T is a protective factor. Surprisingly, our finding is contrary to that of the study in Western country (11). However, the previous studies have demonstrated that the incidence and mortality rate of PTE appears to be significantly deferent in blacks than in whites (13,26,27). It suggested that ethnic background could play an important role in the genetics risk factors involving the development of PTE. The gene polymorphisms of factor V Leiden and coagulation factor II were considered as two main hereditary factors associated with venous thrombosis in Europe and America (28,29). But, their mutations were very rare in Asian populations (30). It showed that the molecular mechanism of PTE may be different in different populations. We postulated that the difference of the gene regulation and expression varies in different populations. However, we couldn’t predict the exact mechanism of different effect of each allele on different populations due to its complexity based on currently available evidence.
According to the results by logistic regression analysis, “complications of DVT or cancer” and “history of operation or injury” were the major risk factors for the development of PTE in a Chinese population. While the factors including oral contraceptive, cigarette smoking, and alcohol drinking weren’t the significant risks for PTE. However, TT genotype of -1654C/T were shown be another independent risk factor for PTE, especially the homozygous carriers of genotype TT of -1654C/T significantly increase the risk of PTE. It must be pointed out that the small number of subjects limited the reliability of the findings. Therefore, a large scale study to verify our preliminary observations in the future is needed.
Conclusions
The SNPs (-1654C/T, -1641A/G and -1476A/T) of protein C promoter gene are associated with susceptibility to PTE in a Chinese population. Especially, the homozygous carriers of genotype TT increase the risk of PTE in a Chinese population. This suggested that a Chinese population has distinct genetics features related to susceptibility to PTE. However, our results should be interpreted with caution. Confirmation of our preliminary observations in a larger scale study is needed.
Acknowledgements
This work was supported by the project of Shanghai Science Committee (No. 074119611, 11411951302 and 114119a3000).
Disclosure: The authors declare no conflict of interest.
References
- Avdonin PV, Kirienko AI, Kozhevnikova LM, et al. C677T mutation in methylentetrahydrofolatereductase gene in patients with venous thromboses from the central region of Russia correlates with a high risk of pulmonary artery thromboembolism. Ter Arkh 2006;78:70-6. [PubMed]
- Cho KH, Jeong MH, Sim DS, et al. Pulmonary thromboembolism due to severe hyperhomocysteinemia associated with a methyltetrahydrofolate reductase mutation. Korean J Intern Med 2013;28:112-5. [PubMed]
- Dunn ST, Trong S. Evaluation of role of factor V Leiden mutation in fatal pulmonary thromboembolism. Thromb Res 1998;91:7-14. [PubMed]
- Küpeli E, Cengiz C, Cila A, et al. Hyperhomocysteinemia due to pernicious anemia leading to pulmonary thromboembolism in a heterozygous mutation carrier. Clin Appl Thromb Hemost 2008;14:365-8. [PubMed]
- Qin J, Dai J, Xu Z, et al. Genetic polymorphism of NOS3 with susceptibility to deep vein thrombosis after orthopedic surgery: a case-control study in Chinese Han population. PLoS One 2013;8:e70033. [PubMed]
- Oguzulgen IK, Yilmaz E, Demirtas S, et al. The role of plasminogen activator inhibitor-1 polymorphism, factor-V-Leiden, and prothrombin-20210 mutations in pulmonary thromboembolism. Clin Appl Thromb Hemost 2009;15:73-7. [PubMed]
- Ro A, Hara M, Takada A. The factor V Leiden mutation and the prothrombin G20210A mutation was not found in Japanese patients with pulmonary thromboembolism. Thromb Haemost 1999;82:1769. [PubMed]
- Seki T, Okayama H, Kumagai T, et al. Arg506Gln mutation of the coagulation factor V gene not detected in Japanese pulmonary thromboembolism. Heart Vessels 1998;13:195-8. [PubMed]
- Aiach M, Nicaud V, Alhenc-Gelas M, et al. Complex association of protein C gene promoter polymorphism with circulating protein C levels and thrombotic risk. Arterioscler Thromb Vasc Biol 1999;19:1573-6. [PubMed]
- Ekim N, Oguzulgen IK, Demir N, et al. The role of angiotensin-converting enzyme gene polymorphism in pulmonary thromboembolism. Thromb Haemost 2004;92:432-3. [PubMed]
- Spek CA, Koster T, Rosendaal FR, et al. Genotypic variation in the promoter region of the protein C gene is associated with plasma protein C levels and thrombotic risk. Arterioscler Thromb Vasc Biol 1995;15:214-8. [PubMed]
- Spek CA, Poort SR, Bertina RM, et al. Determination of the allelic and haplotype frequencies of three polymorphisms in the promoter region of the human protein C gene. Blood Coagul Fibrinolysis 1994;5:309-11. [PubMed]
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med 2003;163:1711-7. [PubMed]
- Worsley DF, Alavi A. Comprehensive analysis of the results of the PIOPED Study. Prospective Investigation of Pulmonary Embolism Diagnosis Study. J Nucl Med 1995;36:2380-7. [PubMed]
- Ohwada A, Takahashi H, Uchida K, et al. Gene analysis of heterozygous protein C deficiency in a patient with pulmonary arterial thromboembolism. Am Rev Respir Dis 1992;145:1491-4. [PubMed]
- Takeda K, Kumagai H, Hayashi S, et al. A case of congenital protein C deficiency with pulmonary thromboembolism. Nihon Kyobu Shikkan Gakkai Zasshi 1994;32:497-501. [PubMed]
- Kogure S, Makita K, Saitoh Y, et al. Anesthetic management of a patient with protein C deficiency associated with pulmonary thromboembolism. Masui 1998;47:831-4. [PubMed]
- Ribeiro A, Correia A, Fernandes F, et al. Pulmonary thromboembolism in a female with resistance to activated protein C. Rev Port Cardiol 1999;18:601-7. [PubMed]
- Taniyasu N, Akiyama K, Takazawa A, et al. Surgical treatment for chronic pulmonary thromboembolism in a patient with protein C deficiency. Kyobu Geka 2001;54:237-40. [PubMed]
- Yoshimura S, Nishimura Y, Funada Y, et al. Pulmonary thromboembolism associated with familial protein C deficiency type I. Nihon Kokyuki Gakkai Zasshi 2003;41:451-6. [PubMed]
- Bhargava K, Chandra N, Omar AK, et al. Protein C deficiency leading to pulmonary thromboembolism in a patient with hereditary spherocytosis. Indian Heart J 2006;58:444-6. [PubMed]
- Yoshida M, Mukohara N, Obo H, et al. Pulmonary thromboendarterectomy for chronic pulmonary thromboembolism in protein C deficiency. Jpn J Thorac Cardiovasc Surg 2006;54:70-4. [PubMed]
- Takami H, Fukushima K, Takizawa M, et al. Protein C deficiency manifested as pulmonary aretery thromboembolism induced by oral contraceptive. Nihon Naika Gakkai Zasshi 2007;96:341-3. [PubMed]
- Chun C, Yang W, Xueding C, et al. Resveratrol downregulates acute pulmonary thromboembolism-induced pulmonary artery hypertension via p38 mitogen-activated protein kinase and monocyte chemoattractant protein-1 signaling in rats. Life Sci 2012;90:721-7. [PubMed]
- Isoda S, Kimura T, Nishimura K, et al. A Case Report of Pulmonary Thromboendarterectomy for Chronic Thromboembolism in a Patient with Protein C Deficiency. Ann Thorac Cardiovasc Surg 2013. [Epub ahead of print]. [PubMed]
- Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: racial contrasts in incidence and in-hospital case fatality. J Natl Med Assoc 2006;98:1967-72. [PubMed]
- Kabrhel C, Varraso R, Goldhaber SZ, et al. Physical inactivity and idiopathic pulmonary embolism in women: prospective study. BMJ 2011;343:d3867. [PubMed]
- Rosendaal FR, Doggen CJ, Zivelin A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost 1998;79:706-8. [PubMed]
- De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med 1999;341:801-6. [PubMed]
- Lu Y, Zhao Y, Liu G, et al. Factor V gene G1691A mutation, prothrombin gene G20210A mutation, and MTHFR gene C677T mutation are not risk factors for pulmonary thromboembolism in Chinese population. Thromb Res 2002;106:7-12. [PubMed]


