
Inflammation and coagulation following minimally invasive extracorporeal circulation technologies
Introduction
Minimally invasive extracorporeal circulation technologies (MiECT) include a number of interventions aimed to reduce the clinical impact of cardiopulmonary bypass (CPB) and to consequently contain postoperative morbidity and mortality. In the past, different kind of CPB circuits were defined as “minimally invasive CPB”, creating a confused scenario. Recently, the definition of a minimally invasive extracorporeal circulation (MiECC) system was standardized (1). A MiECC system must include: (I) a closed CPB circuit; (II) biologically inert blood contact surfaces; (III) reduced priming volume; (IV) a centrifugal pump; (V) a membrane oxygenator; (VI) a heat exchanger; (VII) a cardioplegia system; (VIII) a venous bubble trap/venous air removing device; and (IX) a shed blood management system.
Some of these items, and namely closed, biocompatible circuits with shed blood management have a recognized impact on the inflammatory reaction to CPB and on the hemostatic system activation during cardiac operations. The present review analyzes the mechanisms underlying the effects of MiECT on inflammation, coagulation, and the related clinical outcomes.
Hemostatic system activation during standard CPB and MiECC
Thrombin generation
Despite heparin, thrombin is extensively formed during CPB (2). Thrombin is generated via the intrinsic (contact phase activation) and extrinsic [tissue factor (TF)] pathways; the first is triggered by contact with foreign materials of CPB (material-dependent blood activation) and the second by TF released by the surgical damage to tissues (material-independent blood activation). However, the material-dependent activation is responsible for minor degrees of thrombin generation (3), whereas the material-independent activation is a powerful source of thrombin generation (4).
Two of the main characteristics of a MiECC may impact on thrombin generation during CPB. The first is the presence of a biocompatible coating and reduced foreign surface: biocompatible circuits limit the material-dependent thrombin generation (5). The second (and probably most important) is the closed nature of the MiECC circuit with shed-blood management system, which impacts on the material-independent thrombin generation. TF is extensively released during surgery: both soluble and cell-bound TF concentrations increase during cardiac surgery, especially being expressed by the epicardium, endocardium, adventitia, and sternal bone (2,6).
TF accumulated in the pericardial space is suctioned and re-admitted to the systemic circulation in open CPB circuits. MiECC circuits do not allow re-infusion of shed blood without a previous washing process with cell-savers, and therefore limit thrombin generation via the extrinsic pathway. Previous studies have demonstrated that closed circuits with separation of surgical field suction or elimination of cardiotomy suction induce a significant reduction in thrombin generation markers (4,7,8).
Limiting thrombin generation during CPB is not only beneficial in terms of a reduced blood activation, but even in terms of coagulation factors preservation. A continuously activated thrombin generation creates an alteration in the hemostatic balance after heparin antagonization with protamine: on one side, there are large amounts of thrombin which may trigger clot formation and thrombotic complications; on the other, coagulation factors are reduced in concentration and may be responsible for postoperative bleeding (Figure 1).
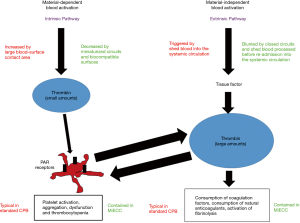
Not surprisingly, different studies addressed the impact of MiECC on markers of the hemostatic system activation and point-of-care coagulation tests. Rahe-Meyer (9) compared a MiECC with a standard CPB circuit testing the whole blood coagulation with thromboelastometry, but he could not find any significant difference during and after CPB. Conversely, Zeitani and associates (10) found shorter coagulation times at thromboelastography in MiECC-treated patients, and reduced levels of prothrombin fragment 1.2 (a thrombin generation marker). This reduction in thrombin generation was recently confirmed by Paparella and associates (11), together with a limitation of fibrinolysis assessed by reduced levels of plasmin-antiplasmin complexes. Anastasiadis and associates (12) found shorter prothrombin time and activated partial thromboplastin time in MiECC-treated patients, which may be linked to a reduced factors consumption.
Overall, there is a concordant scientific information on the beneficial properties of MiECC in reducing thrombin generation, fibrin generation, fibrinolysis.
Platelet activation
The reaction of platelets to CPB is complex and multifactorial. Platelets represent a complex structure which is able to react to a number of stimuli, to interact with the endothelium and sub-endothelial space (adhesion); to interact each other through fibrin links (aggregation); to bind heparin and heparinoids; and finally to release a number of active mediators.
Within the context of the “dynamic” interpretation of the hemostatic process, platelets play a pivotal role not only as fundamental components of the final clot, but even in the process of thrombin generation (13). The early phase of the hemostatic process (initiation) starts with TF release from an injured endothelial surface. This produces a limited amount of thrombin, which in turn promotes platelet activation through the protease-activated receptors (PAR) on the platelet surface (amplification). This thrombin mediated platelet activation generates, on the platelet’s surface, a burst of thrombin generation (propagation) that is able to promote the conversion of fibrinogen into fibrin, which (with the contribution of factor XIII) cross-links platelets through their receptor GPIIb/IIIa finally generating a stable clot.
This dynamic context may be easily disrupted in presence of large amounts of TF. The model of standard CPB, with large amounts of shed blood re-admitted to the systemic circulation without washing out TF and other active mediators is a typical model of artificial alteration of the dynamic hemostatic balance; however, other models are present in nature. Activated monocytes produce TF (blood-borne TF) during sepsis and inflammation, leading again to thrombin generation and platelet activation.
As already pointed out, the model of MiECC is linked to a relatively low thrombin generation, and therefore platelet activation should be better contained. There is a limited amount of studies on platelet count and activation in MiECC. In 2002, Fromes and associates (14) demonstrated that patients treated with MiECC showed a better preservation of platelet count than patients treated with standard CPB. Platelet activation (assessed by beta-thromboglobulin levels) showed slightly lower platelet activation in the MiECC group at all times of CPB. von Willebrand factor activity was reduced in MiECC with respect to standard CPB in a study from Wippermann and associates (15). A study based on multiple electrode aggregometry (9) found a better preservation of platelet function during and after CPB in MiECC-treated patients., and better-preserved platelet counts were identified by Anastasiadis and associates (12).
The effects of standard and reduced heparin anti-coagulation
The evidence of a reduced thrombin generation during MiECC introduces the possibility to modify the standard anticoagulation protocol. Actually, heparin is used during CPB to neutralize the pro-coagulant effects of thrombin, by enhancing the natural binding of antithrombin to thrombin. This effect is generally achieved with an unfractionated heparin (UFH) loading dose of 300–400 IU/kg, followed by additional bolus doses to reach and maintain an activated clotting time (ACT) >450–480 seconds. Theoretically, in presence of a low thrombin generation, lower doses of UFH and a shorter ACT may be sufficient to avoid thrombosis of the CPB circuit. This approach was followed by different authors many years before the MiECC concept was defined and applied in many institutions. In the 1990s, Ovrum and associates conducted studies on reduced heparinization (UFH loading dose 100 IU/kg and target ACT >250 seconds) using heparin-bonded circuits demonstrating the feasibility of this technique (16,17). However, tip-to-tip heparin bonding was the only change that they applied to a conventional CPB, and shed blood was freely re-admitted to the systemic circulation. Conversely, the group of Aldea (18) excluded the cardiotomy suction from the circuit, creating a model which could be considered the ancestor of modern MiECC. Within this model, systemic heparinization was again reduced, resulting in fewer complications and namely less thromboembolic events.
Lately, our group demonstrated that this approach results in a preservation of the circulating antithrombin levels (19), and subsequently proposed a closed system with separation of shed blood, a collapsible venous reservoir, a biocompatible treatment (phosphorylcholine coating), a centrifugal pump and reduced systemic heparinization (UFH loading dose 150 IU/kg and target ACT >300 seconds) (20).
Even in recent years, feasibility of reduced systemic heparinization with target ACT of 250–300 seconds was confirmed in the context of MiECC (21). However, the real effects of this strategy in improving the clinical outcome remain to be established.
Hemodilution and coagulation
One of the pillars of MiECC is the reduction of pump prime volume. This results in a reduced hemodilution during and after CPB. The hemostatic system is certainly affected by the quantity and quality of hemodiluting fluids. Many studies addressed the impact of hemodilution on viscoelastic tests. In an interesting study on cardiac surgery patients, Martin and associates (22) could demonstrate that hemodilution with Ringer’s solution was leading to a hypercoagulable state represented by a shortening of the reaction times, whereas hemodilution with 6% hetastarch and normal saline was leading to a hypocoagulable conditions represented by a decreased clot firmness. Other authors confirmed that there is a hemodilution-related hypercoagulation related to a shortening of the reaction times in thromboelastometry and thromboelastography (23,24). Conversely, hemodilution seems to decrease the clot firmness in other studies.
In contrast with this concept, other authors found a general behaviour of hypocoagulation in patients undergoing hemodilution. A meta-analysis from Hartog and associates (25) focused on hydroxyethyl starch dilution could demonstrate a general decrease in clot firmness and variable effects on clotting times. Recently, we could demonstrate in a large series of about 800 cardiac surgery patients that severe hemodilution on CPB induces a significant prolongation of clotting times and reduction of clot firmness (26). Hemodilution decreases platelet marginalization at the endothelial wall surface, reducing the primary hemostatic effect of platelet adhesion.
Overall, it is likely that MiECC-related containment of hemodilution may result in a better control of the pro-hemorrhagic effects of CPB.
Inflammatory reaction during standard CPB and MiECC
The evidence that CPB is responsible for a whole-body inflammatory reaction dates back to the 1970s. In more recent years, a number of technical improvements have been applied to CPB circuits, aimed to blunt the inflammatory reaction. However, even if certainly reduced in terms of clinical outcomes, the pattern of a systemic inflammatory reaction syndrome (SIRS) still remains evident in cardiac surgery patients. CPB is not the sole responsible for SIRS, since patterns of SIRS have been demonstrated even in off-pump coronary surgery; however, much of the pathway leading to SIRS recognize a direct (blood-foreign surface contact activation) or indirect (link between hemostatic system activation and inflammation) link to CPB.
Contact of blood with the foreign surface of CPB circuit and oxygenator triggers activation of factor XII into factors XIIa (which initiates the intrinsic pathway of coagulation) and XIIf (27).
It is factor XIIf which triggers complement activation through the classical pathway, leading to terminal complement complex (TCC) formation, which in turn is responsible for cell membrane attack, lysis, and cellular death (27).
Factor XIIa cleaves prekallikrein to kallikrein, which in turns trigger bradykinin formation from high molecular weight kininogen, leading to vasodilation and increased cell membrane permeability (28). Kallikrein elicits plasmin generation, and plasmin in combination with factor XIIa activates the alternative complement activation pathway, generating the complement fraction C3a and C5a which induce a pro-inflammatory reaction of neutrophils, macrophages, and monocytes (29).
Aside of the contact-phase activation, there is another important source of SIRS during CPB. As already stressed, during standard CPB thrombin is extensively formed, and thrombin activates platelet through their thrombin receptors of the PAR family. Platelets react to this activation by inducing further thrombin generation, and by releasing the pro-inflammatory cytokines interleukin (IL)-6 and IL-8, powerful neutrophil activators.
Considering the above-mentioned mechanisms underlying the CPB-induced SIRS, it can be certainly be hypothesized that MiECC may theoretically at least partially blunt or limit the inflammatory reaction.
The reduced blood-foreign surfaces interface represented by miniaturized circuit could limit the contact-phase activation. To this respect, an even larger role is played by the standard biocompatible coating that is a pre-requisite of a MiECC system. Heparin-bonded surfaces are associated with a containment of the inflammatory reaction to CPB (30-32). Finally, the recognized thrombin generation limiting effect of shed blood separation could reduce the important role of thrombin in triggering the inflammatory reaction.
There are in fact numerous studies demonstrating that MiECC is accompanied by a reduced activation of the inflammatory cascade (Table 1). Fromes and associates (14), in 2002, found that by the end of CPB the levels of IL-6 were significantly lower in the MiECC group than in standard CPB, that the levels of tumor necrosis factor-alpha rised more in the standard CPB group, and that neutrophil elastase release was lower in the MiECC group. Immer and associates (35) showed that MiECC limits the release of IL-6 and TCC. Abdel-Rahman and associates (33) confirmed lower values of neutrophil elastase release and TCC in MiECC patients vs. standard CPB, and Remadi and associates (34) found lower levels of C-reactive protein after 24 and 48 hours from surgery in MiECC patients vs. conventional CPB.
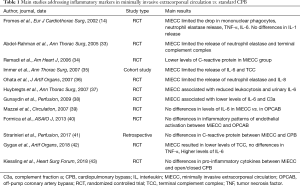
Full table
Other studies found similar results in favor of MiECC vs. standard CPB (36-38). An important randomized controlled study from Mazzei and associates (39) demonstrated that the release of IL-6 in MiECC patients was similar to what measured in off-pump coronary revascularization. The finding that MiECC is comparable to off-pump cardiac surgery in terms of inflammatory reaction was more recently confirmed by Formica and associates (40).
Overall, the majority of the clinical studies confirm the hypothesis that MiECC induces less inflammatory activation than standard CPB, even if different results were observed in other studies (41-43).
The impact of MiECC on coagulation and inflammation-related clinical outcomes
The existing body of scientific literature seems in agreement with the concept that MiECC is associated with a reduced hemostatic system and inflammatory cascade activation. However, from the clinical point of view, a containment in the release of various coagulation and inflammation markers is not “per se” a guarantee of a better outcome. For this reason, different studies investigated coagulation and inflammation-related outcomes, with or without concomitant measure of biochemical markers.
Outcomes associated with the hemostatic system activation may be summarized into four items: perioperative blood loss; need for surgical re-exploration; allogeneic blood products transfusions; and thromboembolic complications.
A lower degree of blood loss (intraoperatively or chest drain postoperatively) was found by Remadi and associates (34) and Gerritsen and associates (44), but this result was not confirmed by Abdel-Rahman and associates, who did not observe any difference in surgical re-exploration rate (33). Conversely, there is a general agreement on a lower rate of allogeneic blood products transfusions in MiECC vs. standard CPB (34,44-46). Thromboembolic complications, and namely stroke and neurologic damage, were found at a lower degree in MiECC patients in two meta-analyses (47,48).
More difficult is the definition of inflammation-related outcomes. Atrial fibrillation is certainly (even if partially) linked to the release of inflammatory markers, and different studies (12,45,49) and meta-analyses (47,48) showed a lower rate of atrial fibrillation in MiECC-treated patients.
Lung function after CPB is another potential inflammatory-related outcome. To this respect, results in the literature are more conflicting. Yilmaz and associates did not find any difference in pulmonary complications between MiECC and conventional CPB (50), whereas Kolat and associates found a lower rate of respiratory insufficiency in MiECC-treated patients (51), and a better postoperative oxygenation was detected by van Boven and associates (46). In general, mechanical ventilation time is shorter in MiECC patients, but this outcome measure reflects many other non-inflammatory related factors.
Other potential outcomes related to the release of inflammatory markers include acute kidney injury and visceral organs complications. However, the multi-factorial nature of these outcomes does not allow to clearly attribute the benefits reported by some authors to the containment of the inflammatory reaction exerted by MiECC.
Conclusions
The evidence that MiECC exerts a beneficial effect in terms of both the hemostatic system activation and the inflammatory cascade is sound. A lower thrombin generation is probably the main factor leading to this pattern, since thrombin is the main hinge between coagulation and inflammation. This is certainly reflected by a better outcome in terms of blood loss and transfusion needs. Less evident, given the multifactorial nature of many inflammation-related complications, is the translation of the limited activation of the inflammatory cascade into a better clinical outcome.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimally invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimally Invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016;22:647-62. [Crossref] [PubMed]
- Edmunds LH, Colman RW. Thrombin generation during cardiopulmonary bypass. Ann Thorac Surg 2006;82:2315-22. [Crossref] [PubMed]
- Boisclair MD, Lane DA, Philippou H. Mechanisms of thrombin generation during surgery and cardiopulmonary bypass. Blood 1993;82:3350-7. [PubMed]
- De Somer F, Van Belleghem Y, Caes F, et al. Tissue factor as the main activator of the coagulation system during cardiopulmonary bypass. J Thorac Cardiovasc Surg 2002;123:951-8. [Crossref] [PubMed]
- Paparella D, Scrascia G, Rotunno C, et al. A biocompatible cardiopulmonary bypass strategy to reduce hemostatic and inflammatory alterations: a randomized controlled trial. J Cardiothorac Vasc Anesth 2012;26:557-62. [Crossref] [PubMed]
- Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Am J Path 1989;134:1087-97. [PubMed]
- Albes JM, Stohr IM, Kaluza M, et al. Physiological coagulation can be maintained in extracorporeal circulation by means of shed blood separation and coating. J Thorac Cardiovasc Surg 2003;126:1504-12. [Crossref] [PubMed]
- Aldea GS, Soltow LO, Chandler WL, et al. Limitation of thrombin generation, platelet activation and inflammation by elimination of cardiotomy suction in patients undergoing coronary artery bypass grafting treated with heparin-bonded circuits. J Thorac Cardiovasc Surg 2002;123:742-55. [Crossref] [PubMed]
- Rahe-Meyer N, Solomon C, Tokuno ML, et al. Comparative assessment of coagulation changes induced by two different types of heart-lung machine. Artif Organs 2010;34:3-12. [Crossref] [PubMed]
- Zeitani J, Buccisano F, Nardella S, et al. Mini-extracorporeal circulation minimizes coagulation abnormalities and ameliorates pulmonary outcome in coronary artery bypass grafting surgery. Perfusion 2013;28:298-305. [Crossref] [PubMed]
- Paparella D, Rotunno C, Guida P, et al. Minimally invasive heart valve surgery: influence on coagulation and inflammatory response. Interact Cardiovasc Thorac Surg 2017;25:225-32. [Crossref] [PubMed]
- Anastasiadis K, Asteriou C, Deliopoulos A, et al. Haematological effects of minimized compared to conventional extracorporeal circulation after coronary revascularization procedures. Perfusion 2010;25:197-203. [Crossref] [PubMed]
- Hoffman M, Munroe DM. A cell-based model of hemostasis. Thromb Haemost 2001;85:958-65. [Crossref] [PubMed]
- Fromes Y, Gaillard D, Ponzio O, et al. Reduction of the inflammatory response following coronary bypass grafting with total minimal extracorporeal circulation. Eur J Cardiothorac Surg 2002;22:527-33. [Crossref] [PubMed]
- Wippermann J, Albes JM, Hartrumpf M, et al. Comparison of minimally invasive closed circuit extracorporeal circulation with conventional cardiopulmonary bypass and with off-pump technique in CABG patients: selected parameters of coagulation and inflammatory system. Eur J Cardiothorac Surg 2005;28:127-32. [Crossref] [PubMed]
- Ovrum E, Holen EA, Tangen G, et al. Completely heparinized cardiopulmonary bypass and reduced systemic heparin: clinical and hemostatic effects. Ann Thorac Surg 1995;60:365-71. [Crossref] [PubMed]
- Ovrum E, Brosstad G, Am Holen E, et al. Effects on coagulation and fibrinolysis with reduced versus full systemic heparinization and heparin-coated cardiopulmonary bypass. Circulation 1995;92:2579-84. [Crossref] [PubMed]
- Aldea GS, Doursounian M, O’Gara P, et al. Heparin-bonded circuits with a reduced anticoagulation protocol in primary CABG: a prospective, randomized study. Ann Thorac Surg 1996;62:410-7; discussion 417-8. [Crossref] [PubMed]
- Ranucci M, Cazzaniga A, Soro G, et al. Antithrombin III saving effect of systemic heparinization and heparin-coated circuits. J Cardiothorac Vasc Anesth 2002;16:316-20. [Crossref] [PubMed]
- Ranucci M, Pazzaglia A, Isgrò G, et al. Closed, phosphorylcoline-coated circuit and reduction of systemic heparinization for cardiopulmonary bypass: the intraoperative ECMO concept. Int J Artif Organs 2002;25:875-81. [Crossref] [PubMed]
- Shapira OM, Korach A, Pinaud F, et al. Safety and efficacy of biocompatible perfusion strategy in a contemporary series of patients undergoing coronary artery bypass grafting - a two-center study. J Cardiothorac Surg 2014;9:196. [Crossref] [PubMed]
- Martin G, Bennett-Guerrero E, Wakeling H, et al. A prospective, randomized comparison of thromboelastographic coagulation profile in patients receiving lactated Ringer’s solution, 6% hetastarch in a balanced-saline vehicle, or 6% hetastarch in saline during major surgery. J Cardiothorac Vasc Anesth 2002;16:441-6. [Crossref] [PubMed]
- Ruttmann TG, James MF, Aronson I. In vivo investigation into the effects of haemodilution with hydroxyethyl starch (200/0.5) and normal saline on coagulation. Br J Anaesth 1998;80:612-6. [Crossref] [PubMed]
- Jones SB, Whitten CW, Despotis GJ, et al. The influence of crystalloid and colloid replacement solutions in acute normovolemic hemodilution: a preliminary survey of hemostatic markers. Anesth Analg 2003;96:363-8. [PubMed]
- Hartog CS, Reuter D, Loesche W, et al. Influence of hydroxyethyl starch (HES) 130/0.4 on hemostasis as measured by viscoelastic device analysis: a systematic review. Intensive Care Med 2011;37:1725-37. [Crossref] [PubMed]
- Ranucci M, Baryshnikova E, Ciotti E, et al. Hemodilution on cardiopulmonary bypass: thromboelastography patterns and coagulation-related outcomes. J Cardiothorac Vasc Anesth 2017;31:1588-94. [Crossref] [PubMed]
- Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009;23:223-31. [Crossref] [PubMed]
- Miller BE, Levy JH. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997;11:355-66. [Crossref] [PubMed]
- Olivencia-Yurvati AH, Mallet RT. Inflammatory response and minimized cardiopulmonary bypass. In: Gourlay T, Gunaydin S. editors. Minimized cardiopulmonary bypass techniques and technologies. Cambridge, UK: Woodhead Publishing, 2012;86-112.
- Yamada H, Kudoh I, Hirose Y, et al. Heparin-coated circuits reduce the formation of TNF alpha during cardiopulmonary bypass. Acta Anaesthesiol Scand 1996;40:311-7. [Crossref] [PubMed]
- Giomarelli P, Naldini A, Biagioli B, et al. Heparin coating of extracorporeal circuits inhibits cytokine release from mononuclear cells during cardiac operations. Int J Artif Organs 2000;23:250-5. [Crossref] [PubMed]
- Olsson C, Siegbahn A, Henze A, et al. Heparin-coated cardiopulmonary bypass circuits reduce circulating complement factors and interleukin-6 in paediatric heart surgery. Scand Cardiovasc J 2000;34:33-40. [Crossref] [PubMed]
- Abdel-Rahman U, Ozaslan F, Risteski PS, et al. Initial experience with a minimized extracorporeal bypass system: is there a clinical benefit? Ann Thorac Surg 2005;80:238-43. [Crossref] [PubMed]
- Remadi JP, Rakotoarivelo Z, Marticho P, et al. Prospective randomized study comparing coronary artery bypass grafting with the new mini-extracorporeal circulation Jostra System or with a standard cardiopulmonary bypass. Am Heart J 2006;151:198. [Crossref] [PubMed]
- Immer FF, Ackermann A, Gygax E, et al. Minimal extracorporeal circulation is a promising technique for coronary artery bypass grafting. Ann Thorac Surg 2007;84:1515-20; discussion 1521. [Crossref] [PubMed]
- Ohata T, Mitsuno M, Yamamura M, et al. Minimal cardiopulmonary bypass attenuates neutrophil activation and cytokine release in coronary artery bypass grafting. J Artif Organs 2007;10:92-5. [Crossref] [PubMed]
- Huybregts RA, Morariu AM, Rakhorst G, et al. Attenuated renal and intestinal injury after use of a minicardiopulmonary bypass system. Ann Thorac Surg 2007;83:1760-6. [Crossref] [PubMed]
- Gunaydin S, Sari T, McCusker K, et al. Clinical evaluation of minimized extracorporeal circulation in high-risk coronary revascularization: impact on air handling, inflammation, hemodilution and myocardial function. Perfusion 2009;24:153-62. [Crossref] [PubMed]
- Mazzei V, Nasso G, Salamone G, et al. Prospective randomized comparison of coronary bypass grafting with minimal extracorporeal circulation system (MECC) versus off-pump coronary surgery. Circulation 2007;116:1761-7. [Crossref] [PubMed]
- Formica F, Mariani S, Broccolo F, et al. Systemic and myocardial inflammatory response in coronary artery bypass graft surgery with miniaturized extracorporeal circulation: differences with a standard circuit and off-pump technique in a randomized clinical trial. ASAIO J 2013;59:600-6. [Crossref] [PubMed]
- Starinieri P, Declercq PE, Robic B, et al. A comparison between minimized extracorporeal circuits and conventional extracorporeal circuits in patients undergoing aortic valve surgery: is ‘minimally invasive extracorporeal circulation’ just low prime or closed loop perfusion? Perfusion 2017;32:403-8. [Crossref] [PubMed]
- Gygax E, Kaeser HU, Stalder M, et al. Type II minimal-invasive extracorporeal circuit for aortic valve replacement: a randomized controlled trial. Artif Organs 2018;42:620-9. [Crossref] [PubMed]
- Kiessling AH, Keller H, Moritz A. Prospective, randomized, un-blinded three arm study in coronary artery revascularization with minimal invasive extracorporeal circulation systems (MiECC): surrogate parameter analysis of biocompatibility. Heart Surg Forum 2018;21:E179-86. [Crossref] [PubMed]
- Gerritsen WB, van Boven WJ, Wesselink RM, et al. Significant reduction in blood loss in patients undergoing minimal extracorporeal circulation. Transfus Med 2006;16:329-34. [Crossref] [PubMed]
- El-Essawi A, Hajek T, Skorpil J, et al. Are minimized perfusion circuits the better heart lung machines? Final results of a prospective randomized multicentre study. Perfusion 2011;26:470-8. [Crossref] [PubMed]
- van Boven WJ, Gerritsen WB, Driessen AH, et al. Minimised closed circuit coronary artery bypass grafting in the elderly is associated with lower levels of organ-specific biomarkers: a prospective randomised study. Eur J Anaesthesiol 2013;30:685-94. [Crossref] [PubMed]
- Zangrillo A, Garozzo FA, Biondi-Zoccai G, et al. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: a meta-analysis of randomized controlled studies. J Thorac Cardiovasc Surg 2010;139:1162-9. [Crossref] [PubMed]
- Biancari F, Rimpilainen R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart 2009;95:964-9. [Crossref] [PubMed]
- Panday GF, Fisher S, Bauer A, et al. Minimal extracorporeal circulation and off-pump compared to conventional cardiopulmonary bypass in coronary surgery. Interact Cardiovasc Thorac Surg 2009;9:832-6. [Crossref] [PubMed]
- Yilmaz A, Sjatskig J, van Boven WJ, et al. Combined coronary artery bypass grafting and aortic valve replacement with minimal extracorporeal closed circuit circulation versus standard cardiopulmonary bypass. Interact CardioVasc Thorac Surg 2010;11:754-7. [Crossref] [PubMed]
- Kolat P, Ried M, Haneya A, et al. Impact of age on early outcome after coronary bypass graft surgery using minimized versus conventional extracorporeal circulation. J Cardiothorac Surg 2014;9:143. [Crossref] [PubMed]


