
Patient-reported outcomes in lung and esophageal cancer
Introduction
The delivery of patient-centered care hinges on the ability to measure and implement into clinical practice what is of greatest concern to patients. Objective data regarding perioperative complications and survival must be complemented with patient-reported outcomes (PROs) to contextualize therapy and outcomes for individual patients. This is of utmost importance in patients with disorders of the lung and esophagus, which carry significant morbidity, in part due to a high burden of symptoms affecting health-related quality of life (HRQOL) (1). This review will focus on the topic of PROs in malignant thoracic disease, including lung and esophageal carcinoma, and highlight key issues regarding integration of PROs in to clinical thoracic surgery research.
Utility of PROs in surgery
PROs are measures of HRQOL, including physical, emotional and mental well-being obtained by patient self-report, without interpretation of their response by clinicians (2). The addition of PROs to traditionally collected outcome measures (i.e., morbidity, mortality, overall survival) can offer a comprehensive overview of the patient experience around the time of surgery.
The patient’s account of his or her health is also crucial in understanding the effect of an intervention and measuring the quality of care being delivered. The impact of an intervention on disability, symptoms and HRQOL is an important outcome, which only patients can assess. As such, patient-centric measures of health status can add meaningfully to the results of prospective studies of comparative effectiveness research involving different treatments and outcomes, and can aid in guideline development (2). In addition, there is a positive relationship between improved HRQOL scores and long-term survival in cancer patients (3). Using PROs to develop better prognostic estimates can inform shared decision making and further accelerate patient-centered care (4). The benefits of PROs also include superior response rates as the onus lies on individual patients as opposed to clinicians that look after multiple patients (2). Additional advantages include reduced observer bias and increased public accountability of health services and health care professionals (2). PROs, therefore, can also play a role in performance measurement and quality improvement (2,5).
Available instruments
Both generic and disease-specific PRO measures (PROMs) are available for use in thoracic surgery. They are often administered concomitantly and can provide complementary information about the patient experience (Table 1). The most commonly used tools in thoracic surgery include the Medical Outcomes Study Short Form 36 (SF-36), the European Organization for Research and Treatment of Cancer (EORTC), and Functional Assessment of Cancer Therapy (FACT) Oncologic and Organ Specific Modules.
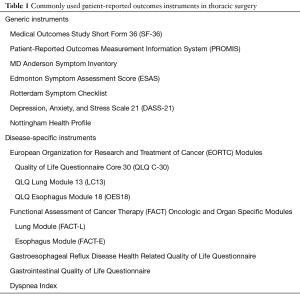
Full table
SF-36 is a well-validated and widely used generic outcome measure (6). The questionnaire consists of 36 items and is grouped into eight scales: physical functioning, social functioning, role limitations caused by physical problems, role limitations caused by emotional problems, mental health, energy/vitality, bodily pain, and general health and a single item concerning health change. These can be further classified into two higher order domains representing the physical and mental aspects of HRQOL (6). EORTC modules consist of a generic Quality of Life Core Questionnaire (QLQ C-30) as well as the disease-specific QLQ Lung Module 13 (LC13) and QLQ Esophagus Module 18 (OES18) (7). The 30-item core questionnaire explores five functional scales (physical, role, emotional, cognitive, social), three symptom scales (fatigue, pain, nausea and vomiting), and a global HRQOL scale. The remainder of the questions cover additional symptoms commonly reported by cancer patients (i.e., dyspnea, sleep disturbance, etc.), and the financial impact of the disease and treatment (7). The modular supplements include both multi-item and single-item measures of lung cancer-associated (i.e., coughing, hemoptysis, dyspnea and pain) and esophageal cancer-associated symptoms (i.e., dysphagia, early satiety and heartburn), as well as side-effects from conventional chemo- and radiotherapy (i.e., hair loss, neuropathy, sore mouth and dysphagia) (8,9). Similarly, the general version of the FACT questionnaire (FACT-G) applies to a variety of chronic illnesses and cancers. It consists of 27 items that explore various facets of HRQOL over 4 subscales: physical, social, emotional and functional well-being (10). The lung and esophageal cancer specific modules (FACT-L and FACT-E, respectively) explore disease specific symptoms including chest pain, cough, xerostomia, weight loss and voice quality (11,12). Domain comparisons of the EORTC and FACT questionnaires have shown discordance among tools, particularly with respect to emotional, social and overall QOL, as well as disease-specific symptomatology. For instance, the social function scale of the EORTC QLQ-C30 has items that differ in content to those within the social function scale of the FACT-G. Although the disease-specific modules contain similar items, differences in scoring systems preclude any meaningful comparisons of results. While both tools have acceptable feasibility and validity, they are not interchangeable and their variable emphasis on different aspects of QOL has affected their clinical utility in patients with lung and esophageal cancer (13). Also of note, the EORTC QLQ-C30 and LC13 modules were validated in a cohort of patients with inoperable lung cancer (7,8); their use therefore, may be limited amongst resectable lung cancer patients and must be guided by the specific aims of the research question at hand.
The recently developed Patient-Reported Outcome Measurement Information System (PROMIS) is another well-validated system of PROMs that includes a variety of questionnaires spanning physical, mental and social health domains (14). PROMIS surveys can be tailored to any patient population and are based on item response theory to adapt to the specific symptoms of a patient (14). PROMIS is now recommended by the Center for Medical Technology Policy—an independent non-profit organization established to improve the quality of healthcare research—as a preferred PRO measure for clinical research and has been used in a variety of fields including thoracic surgery (14,15).
PROs in lung carcinoma
There is a large body of literature on the topic of PROs and HRQOL in lung cancer (15-22). The majority of studies, however, are retrospective or prospective observational studies of limited sample sizes. Despite variations in quality, most reports describe an initial decline in HRQOL, with eventual recovery within 6–12 months of surgery. For instance, in a longitudinal study of PROs using the MD Anderson Symptom Inventory, Fagundes et al. demonstrated that fatigue, pain, dyspnea and disturbed sleep peaked 3–5 days after surgery, with recovery within 3 months of surgery (17). Likewise, in their prospective study of PROs amongst 127 patients undergoing lung cancer surgery, Khullar et al. reported that nearly all QOL scores, including physical function, pain, sleep and fatigue, demonstrated resolution within 6 months of surgery (15). This study also examined the role of PROs as end points of comparative effectiveness research in lung cancer. Patients undergoing video-assisted thoracic surgery (VATS) experienced superior levels of physical function and ability to participate in social activities, as well as less pain intensity and fatigue in relation to those undergoing thoracotomies. No group differences in PROs were identified amongst patients treated with lobectomy vs. sublobar resection (15). These findings were in keeping with previous SF-36 studies by Zhao et al. (19) and Fernando et al. (20), in which minimally invasive thoracoscopy was associated with improved QOL outcomes (including dyspnea, pain, energy and physical role functioning) compared to thoracotomy. In the latter study, segmentectomy was associated with a detriment in dyspnea scores at 2 years from surgery compared to wedge resection (20). The impact of extent of resection on HRQOL is well-documented in the literature. In Balduyck et al.’s study of 30 patients using EORTC questionnaires, sleeve lobectomy was associated with a smaller impairment in physical, role and cognitive functioning, compared to pneumonectomy (21). This finding was later corroborated in a larger population-based study by Sartipy et al. using the SF-36 survey in 117 patients, where lobectomy also had less of a negative impact on physical aspects of HRQOL than pneumonectomy at 6 months (22).
The predictive value of QOL for survival was first demonstrated by Ganz et al. in a sample of 40 patients with advanced metastatic lung cancer, where patients with higher scores had better mortality, and vice versa (23). Since then, numerous studies (24-27) have explored QOL as a prognostic marker in lung cancer, including a large study of 809 patients using the EORTC QLQ-C30 and QLQ-LC13 questionnaires. After adjusting for demographic and clinical predictors of survival, QOL measures of postoperative anxiety, strength, dyspnea and physical function were all found to be independent predictors of long-term overall survival (27). The abovementioned studies demonstrate the feasibility of measuring PROs and their utility as valuable end points in the evaluation and treatment of lung cancer.
PROs in esophageal carcinoma
While historically the surgical esophageal cancer literature has focused on traditional outcomes such as perioperative morbidity and mortality, there has been an increase in the inclusion of PROs as study endpoints in more recent years. PROs and HRQOL are being used in research at all stages of the patient journey, from diagnosis, to treatment response, to long-term survival (28).
At diagnosis, esophageal cancer patients are often symptomatic with dysphagia, weight loss, or chest pain. Because of this late presentation, cancer stage is often advanced. Studying the hypothesis that patient quality of life may be associated with clinical stage, a multi-institutional Canadian study of 135 esophageal cancer patients found that pre-treatment FACT-E scores could distinguish lower and higher clinical T-stage patients (29). Current treatment decisions rely significantly on cancer stage; as the reproducibility and reliability of PROs in determining stage is confirmed, they can become an important adjunct for patients and physicians when planning treatment.
As in lung cancer, PROs are being used to compare interventions in esophageal cancer research. For example, in the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) trial which established current standard of care tri-modality therapy (30) for patients with locally advanced esophageal cancer, HRQOL was included as a secondary endpoint to compare scores in those who received chemoradiotherapy and surgery versus surgery alone. The investigators found HRQOL dropped immediately following chemoradiotherapy, but there was no difference in scores postoperatively, suggesting that preoperative therapy does not impact recovery following surgery (31). This example illustrates how PRO data created a more compelling case to treat patients with tri-modality therapy. Of note, survey response rates were 54% or 76% (31), which highlights the challenge of incorporating HRQOL assessment in clinical and research practice.
The prognostic value of quality of life scores in esophageal cancer has been demonstrated from as early as 2001 (32). Several studies since then have shown both pre- and postoperative HRQOL scores to be associated with survival (33-37); however, no prognostic tools currently available for survival prediction in esophageal cancer incorporate these measures (38).
Other areas of investigation with PROs in esophageal cancer include change in scores during therapy and time to return to baseline following treatment (39). Many opportunities exist to enhance research with the addition of this outcome, and research groups around the world are moving toward routine collection of PRO data in esophageal cancer databases and studies.
Future directions—challenges and opportunities
The numerous advantages of PROs are balanced by several challenges, which may limit their rapid integration in to clinical practice. First, most PROMs are designed by physicians, leading to subjective variability in the content and scoring systems. For this reason, the US Food and Drug Administration (FDA) now mandates open-ended patient input in the development of all PROMs (40). Second, accurate measurement of QOL hinges on highly variable patient factors, including language and culture. Further, the poor survival of thoracic malignancies and rapid deterioration of performance status due to disease and treatment related symptoms poses a challenge for long-term follow-up and serial HRQOL measurement (41). However, high morbidity and short survival is precisely why the inclusion of PROs is crucial in studies evaluating treatments for lung and esophageal cancer. Numerous national societies, including the American College of Chest Physicians (ACCP) and the FDA, now endorse the routine use of PROs to inform clinical guidelines and as end points in clinical trials (1). Global interest in PROMs has encouraged national agencies to standardize approaches to collecting and reporting PROs. This includes the Canadian Institute for Health Information’s (CIHI) PROMs program, launched to support the development of PROMs data collection standards and reporting in priority topics (42). The US National Institutes of Health-sponsored PROMIS tool is another example of a national platform for the measurement of patient-reported symptoms and other health outcomes, and is available online. Linkage of PRO data with clinical registry data (i.e., Society of Thoracic Surgeons’ National Database) has been tested successfully in thoracic surgery, allowing for more research with patient-centric outcomes (15). To further enhance the uptake of value-based health care, the International Consortium for Health Outcomes Measurement (ICHOM) has proposed a core set of health outcomes, gathered using the expert opinion of clinicians and patient representatives, for collection in routine clinical practice internationally. This includes recommendations for lung cancer, which focus on 5 overarching themes including degree of health, survival and quality of death (43).
Conclusions
PROs are central to the delivery of high-quality patient-centered care. Patients with lung and esophageal cancer are vulnerable to significant detriments in HRQOL as a result of disease and treatment, which underscores the importance of including PROs as important outcomes in research. There is a paucity of high-quality studies focusing on PROs in malignant thoracic disease. Incorporation of PROs into outcomes research is expected to facilitate the delivery of value-based surgical care for patients with lung and esophageal cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Khullar OV, Fernandez FG. Patient-Reported Outcomes in Thoracic Surgery. Thorac Surg Clin 2017;27:279-90. [Crossref] [PubMed]
- Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346:f167. [Crossref] [PubMed]
- Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009;7:102. [Crossref] [PubMed]
- Chang VT, Scott CB, Gonzalez ML, et al. Patient-Reported Outcomes for Determining Prognostic Groups in Veterans With Advanced Cancer. J Pain Symptom Manage 2015;50:313-20. [Crossref] [PubMed]
- Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, et al. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q 2014;92:754-75. [Crossref] [PubMed]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30A:635-42. [Crossref] [PubMed]
- Malmström M, Klefsgard R, Ivarsson B, et al. Quality of life measurements as an indicator for timing of support after oesophagectomy for cancer: a prospective study. BMC Health Serv Res 2015;15:96. [Crossref] [PubMed]
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. [Crossref] [PubMed]
- Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. [Crossref] [PubMed]
- Darling G, Eton DT, Sulman J, et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer 2006;107:854-63. [Crossref] [PubMed]
- Blazeby JM, Kavadas V, Vickery CW, et al. A prospective comparison of quality of life measures for patients with esophageal cancer. Qual Life Res 2005;14:387-93. [Crossref] [PubMed]
- Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol 2007;25:5106-12. [Crossref] [PubMed]
- Khullar OV, Rajaei MH, Force SD, et al. Pilot Study to Integrate Patient Reported Outcomes After Lung Cancer Operations Into The Society of Thoracic Surgeons Database. Ann Thorac Surg 2017;104:245-53. [Crossref] [PubMed]
- Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol 2008;26:233-41. [Crossref] [PubMed]
- Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg 2015;150:613-9.e2. [Crossref] [PubMed]
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [Crossref] [PubMed]
- Zhao J, Zhao Y, Qiu T, et al. Quality of life and survival after II stage nonsmall cell carcinoma surgery: Video-assisted thoracic surgery versus thoracotomy lobectomy. Indian J Cancer 2015;52 Suppl 2:e130-3. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg 2015;149:718-25; discussion 725-6. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [Crossref] [PubMed]
- Sartipy U. Prospective population-based study comparing quality of life after pneumonectomy and lobectomy. Eur J Cardiothorac Surg 2009;36:1069-74. [Crossref] [PubMed]
- Ganz PA, Lee JJ, Siau J. Quality of life assessment. An independent prognostic variable for survival in lung cancer. Cancer 1991;67:3131-5. [Crossref] [PubMed]
- Langendijk H, Aaronson NK, de Jong JM, et al. The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol 2000;55:19-25. [Crossref] [PubMed]
- Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol 2009;27:5816-22. [Crossref] [PubMed]
- Möller A, Sartipy U. Associations between changes in quality of life and survival after lung cancer surgery. J Thorac Oncol 2012;7:183-7. [Crossref] [PubMed]
- Yun YH, Kim YA, Sim JA, et al. Prognostic value of quality of life score in disease-free survivors of surgically-treated lung cancer. BMC Cancer 2016;16:505. [Crossref] [PubMed]
- Alghamedi A, Buduhan G, Tan L, et al. Quality of life assessment in esophagectomy patients. Ann Transl Med 2018;6:84. [Crossref] [PubMed]
- Kidane B, Ali A, Sulman J, et al. Health-related quality of life measure distinguishes between low and high clinical T stages in esophageal cancer. Ann Transl Med 2018;6:270. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Noordman BJ, Verdam MGE, Lagarde SM, et al. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial. J Clin Oncol 2018;36:268-75. [Crossref] [PubMed]
- Blazeby JM, Brookes ST, Alderson D. The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut 2001;49:227-30. [Crossref] [PubMed]
- Djarv T, Metcalfe C, Avery KN, et al. Prognostic value of changes in health-related quality of life scores during curative treatment for esophagogastric cancer. J Clin Oncol 2010;28:1666-70. [Crossref] [PubMed]
- Djarv T, Lagergren P. Six-month postoperative quality of life predicts long-term survival after oesophageal cancer surgery. Eur J Cancer 2011;47:530-5. [Crossref] [PubMed]
- Kidane B, Sulman J, Xu W, et al. Baseline measure of health-related quality of life (Functional Assessment of Cancer Therapy-Esophagus) is associated with overall survival in patients with esophageal cancer. J Thorac Cardiovasc Surg 2016;151:1571-80. [Crossref] [PubMed]
- Quinten C, Martinelli F, Coens C, et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014;120:302-11. [Crossref] [PubMed]
- Kidane B, Sulman J, Xu W, et al. Pretreatment quality-of-life score is a better discriminator of oesophageal cancer survival than performance status. Eur J Cardiothorac Surg 2017;51:148-54. [Crossref] [PubMed]
- Gupta V, Coburn N, Kidane B, et al. Survival prediction tools for esophageal and gastroesophageal junction cancer: A systematic review. J Thorac Cardiovasc Surg 2018;156:847-56. [Crossref] [PubMed]
- Trudel JG, Sulman J, Atenafu EG, et al. Longitudinal Evaluation of Trial Outcome Index Scores in Patients With Esophageal Cancer. Ann Thorac Surg 2016;102:269-75. [Crossref] [PubMed]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research. U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research. U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [Crossref]
- Anant M, Guleria R, Pathak AK, et al. Quality of life measures in lung cancer. Indian J Cancer 2005;42:125-32. [Crossref] [PubMed]
- Dudgeon D. The Impact of Measuring Patient-Reported Outcome Measures on Quality of and Access to Palliative Care. J Palliat Med 2018;21:S76-80. [Crossref] [PubMed]
- Mak KS, van Bommel AC, Stowell C, et al. Defining a standard set of patient-centred outcomes for lung cancer. Eur Respir J 2016;48:852-60. [Crossref] [PubMed]


