
Hippo pathway as another oncogenic mediator to promote immune evasion by PD-L1 signaling
The programmed cell death protein (PD)-1 and programed death ligand 1 (PD-L1) based immunotherapeutic agent is a cornerstone for the treatment of cancer. The expression of PD-L1 was developed as a biomarker to predict the efficacy of anti-PD1/PD-L1 pathway based immune check point inhibitor. In addition, increased PD-L1 in tumor and tumor microenvironment could be considered as representative biomarkers for immune suppressive microenvironment. The expression of PD-L1 is known to be increased by innate immune resistance as well as acquired immune resistance (1). In acquired immune resistance, the expression of PD-L1 secondarily increased by interferon (IFN) gamma secreted by immune cells including cytotoxic T cells in inflamed tumor, but in case of innate immune resistance the expression of PD-L1 increased directly by oncogenic signaling pathway which might be not related to inflamed tumor microenvironment (2). The expression of PD-L1 may not predict the effect of the anti-PD1 pathway based immune check point inhibitor in case of PD-L1 of tumors directly elevated by the oncogenic signal pathway, however the increased PD-L1 itself is likely to induce immune resistance in tumor. The regulation of PD-L1 by oncogenic signaling pathway already has been known in various type of cancer including non-small cell lung cancer (3-7). Activation of common oncogenic signaling pathways such as EGFR, EML4-ALK, JAK/STAT, RAS/ERK, or PI3K/AKT/MTOR has been shown to affect tumoral PD-L1 expression (3-6). The regulation of PD-L1 expression by oncogenic signaling pathway can act either directly on specific target sequence of PD-L1 or indirectly mediated by the activation of transcription factors. Molecules as IRF-1, STAT3, STAT1, c-Jun, HIFs, or NF-κB as a transcription factor can migrate to the nucleus, bind to specific sequence of promoter of PD-L1 to induce its expression (7).
Recently, Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ) have been suggested as one of the transcriptional regulators of PD-L1. Janse van Rensburg et al. demonstrate that YAP/TAZ of the Hippo pathway can modulate the immune response by transcriptional regulation of PD-L1 in lung cancer and breast cancer cell lines (8). The Hippo pathway (also known as the Salvador-Warts-Hippo pathway) is conserved between species as a regulator of organ growth and tissue homeostasis during injury (9). The Hippo pathway consists of a large network of proteins. The mammalian STE20-like protein kinase 1 (MST1)/MST2 and large tumor suppressor 1 (LAST1)/LAST2 together with the adaptor proteins Salvador homologue 1, MOB activator 1A (MOB1A)/MOB1B phosphorylates YAP and TAZ, leading to their cytoplasmic localization and that also induce their ubiquitin-mediated proteolysis (9). YAP and TAZ which translocated to nucleus promoting cell growths and tumorigenesis with transcriptional co-activator TEA domain family members (TEADs) (9).
In various types of cancers such as lung, colorectal, ovarian, liver and prostate cancer, the deregulation of the Hippo pathway has been reported. YAP1 overexpression has been reported in 60–70% non-small cell carcinoma (NSCLC) cases (10-12). Activation of YAP in lung cancer is reported to be associated with high T, N and M stages, lymphovascular invasion, and poor prognosis (11,12). In vivo study, YAP and TAZ are required for normal lung development and homeostasis, however, in adults YAP and TAZ are not required. In lung adenocarcinoma transgenic mice models, genetic loss of YAP strongly reduces tumors formation in the KrasG12D:Lkb1L/L mouse, constitutive expression of TAZ induces immortalized bronchial cells to form tumors in immunocompromised mice, and ectopic YAP expression can induce small lung adenomas to progress high-grade adenocarcinoma in the KrasG12D knock-in mouse model (13,14). In EGFR mutant lung cancer, enhanced YAP expression has been reported to cause EGFR TKI resistance in EGFR mutated NSCLC. YAP is reported to increase Erbb3 to promote EGFR-TKI resistant EGFR mutant lung cancer cells or activates AXL tyrosine kinase which induced PI3K/AKT signaling to promote the EGFR-TKI resistance (15,16).
Janse van Rensburg et al. analyzed immune-related gene signature regulated by TAZ and YAP in breast cancer cells using NanoString gene expression profiling (8). They demonstrated that TAZ or YAP overexpression in MCF10A induced significant changes in the mRNA expression of 24 genes such as S1PR1, NLRP3, CXCR4, CFI and the immune checkpoint molecules PD-L1. Overexpression of TAZ (or knockdown of MST1/2 or LATS1/2) increased PD-L1 expression in TAZ-low/PD-L1-low cell lines and loss of TAZ (or LATS1/2 overexpression) reduced PD-L1 in TAZ-high/PD-L1-high cell lines. They demonstrated that TAZ directly interacts with the PD-L1 promoter through the TEAD family transcription factor. A deletion scan of the PD-L1 promoter identified a putative TEAD-response element. As functionally, cancer cells with induced TAZ expression enhanced T cell apoptosis via PD-L1 mediated and suppressed T cell IL-2 production. Blocking of PD-L1 suppressed apoptosis of T cells and rescued T cell IL-2 production when cocultured with TAZ over expressing lung cancer cells (Figure 1).
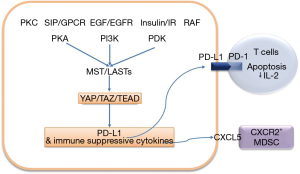
Previous studies also have been suggested that YAP and TAZ are involved in cancer immune evasion by PD-L1 transcriptional regulation. YAP was reported to regulate the transcription of PD-L1 through direct YAP/TAZ/TEAD complex binding to the PD-L1 promoter in EGFR-TKI resistant EGFR mutated lung cancer cell line (17). They performed a chromatin immunoprecipitation assay in YAP over-expressed EGFR mutant PC9 cells and confirmed YAP/TEAD binds to the PD-L1 promoter region (17). Miao et al. also reported that PD-L1 transcriptional regulation by YAP/TEAD in other lung cancer cells lines with high PD-L1, such as EGFR wild type H460, SKLU-1 and H1299 (18). Thus, YAP and TAZ transcriptional regulation of PD-L1 was considered to be confirmative.
In addition, authors also demonstrated that PD-L1 expression by YAP and TAZ is affected by many oncogenic signaling pathway, as upstream regulator of the Hippo pathway such as EGFR/PI3K, G-protein coupled receptor (GPCR), protein kinase C, 3-phosphoinositidedependent protein kinase 1 (PDK1) and RAF (8). They identified many oncogenic signaling pathways converge to YAP/TAZ and affect the expression of PD-L1 by YAP/TAZ transcriptional regulation (Figure 1).
However, authors did not demonstrate a TEAD response element in mouse PD-L1 promoter. In addition, multiple candidate TAZ targets identified in human NanoString gene expression profile screen were not upregulated in mouse cell lines (8). This suggests that there may be broader species-specific differences in the TAZ transcriptional program. It has not been previously identified and emphasizes the importance of a selection in vivo model during tumor immunology research.
Janse van Rensburg et al. suggested that YAP and TAZ are not only an important oncogenic pathway regulator, but also regulates PD-L1 expression, probably promotes an immune suppressive microenvironment and might be a predictive marker of immune check point inhibitor. The Oncogenic signaling pathway has been known to induce changes in immune microenvironment through cross-talk of cytokine/chemokine network as well as PD-L1 expression in tumors (2). However, there is still insufficient evidence to suggest that such changes in immune microenvironment and accompanied increase in tumor PD-L1 could be considered as predictive biomarkers of the immune checkpoint inhibitor therapy.
YAP and TAZ also has been known to promote immune suppressive microenvironment through cytokine/chemokine network. YAP was reported to induce CXCL5 upregulation of tumor cells in prostate cancer models and CXCL5 attracted CXCR2-expressing myeloid-derived suppressor cells (MDSCs) (19). YAP also was reported to modulate multiple cytokines/chemokines, which in turn induced the differentiation and accumulation of MDSCs in Kras:p53 mutant pancreatic cancer in vivo mice model (20).
Based on the effects of YAP and TAZ which can promote immune suppressive microenvironment through chemokine/cytokine network, further studies in analysis of immune microenvironment which should be sophisticatedly selected in vivo model are needed. In addition, effects of immune microenvironment through upstream oncogenic regulator of YAP and TAZ also should be analyzed.
In conclusion, authors clearly demonstrate the transcriptional regulation of PD-L1 by YAP and TAZ. This PD-L1 upregulation through TAZ/TEAD induce T cell apoptosis. They also suggest that immune gene signature regulated by YAP and TAZ appears to have interspecies specificity. Nowadays, the role of YAP and TAZ on crosstalk between immune microenvironment and tumor recently has been suggested. Thus additional studies focused on effects of Hippo-YAP/TAZ to immune microenvironment are strongly encouraged.
Acknowledgements
Funding: This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the 2016 Ministry of Education (2016R1D1A1A02937400).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018;48:399-416. [Crossref] [PubMed]
- Jiang X, Zhou J, Giobbie-Hurder A, et al. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013;19:598-609. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- Koh J, Jang JY, Keam B, et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1alpha and STAT3. Oncoimmunology 2015;5:e1108514. [Crossref] [PubMed]
- Lastwika KJ, Wilson W 3rd, Li QK, et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res 2016;76:227-38. [Crossref] [PubMed]
- Zerdes I, Matikas A, Bergh J, et al. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene 2018;37:4639-61. [Crossref] [PubMed]
- Janse van Rensburg HJ, Azad T, Ling M, et al. The Hippo Pathway Component TAZ Promotes Immune Evasion in Human Cancer through PD-L1. Cancer Res 2018;78:1457-70. [Crossref] [PubMed]
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014;94:1287-312. [Crossref] [PubMed]
- Guo J, Wu Y, Yang L, et al. Repression of YAP by NCTD disrupts NSCLC progression. Oncotarget 2017;8:2307-19. [PubMed]
- Kim JM, Kang DW, Long LZ, et al. Differential expression of Yes-associated protein is correlated with expression of cell cycle markers and pathologic TNM staging in non-small-cell lung carcinoma. Hum Pathol 2011;42:315-23. [Crossref] [PubMed]
- Wang Y, Dong Q, Zhang Q, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 2010;101:1279-85. [Crossref] [PubMed]
- Lau AN, Curtis SJ, Fillmore CM, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J 2014;33:468-81. [Crossref] [PubMed]
- Zhang W, Gao Y, Li F, et al. YAP promotes malignant progression of Lkb1-deficient lung adenocarcinoma through downstream regulation of survivin. Cancer Res 2015;75:4450-7. [Crossref] [PubMed]
- Hsu PC, You B, Yang YL, et al. YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget 2016;7:51922-33. [Crossref] [PubMed]
- Lee JE, Park HS, Lee D, et al. Hippo pathway effector YAP inhibition restores the sensitivity of EGFR-TKI in lung adenocarcinoma having primary or acquired EGFR-TKI resistance. Biochem Biophys Res Commun 2016;474:154-60. [Crossref] [PubMed]
- Lee BS, Park DI, Lee DH, et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun 2017;491:493-9. [Crossref] [PubMed]
- Miao J, Hsu PC, Yang YL, et al. YAP regulates PD-L1 expression in human NSCLC cells. Oncotarget 2017;8:114576-87. [Crossref] [PubMed]
- Wang G, Lu X, Dey P, et al. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov 2016;6:80-95. [Crossref] [PubMed]
- Murakami S, Shahbazian D, Surana R, et al. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene 2017;36:1232-44. [Crossref] [PubMed]


