
Re-challenge chemotherapy in patients with sensitive relapse small-cell lung cancer and interstitial lung disease
Introduction
Small-cell lung cancer (SCLC) accounts for approximately 13% of all cases of lung cancers (1). Despite the efficacy of platinum-based chemotherapy as the main treatment for SCLC, most patients will develop disease relapse or progression (2). Relapsed SCLC can be classified into two main groups on the basis of the treatment-free interval (TFI) after the completion of first-line chemotherapy: sensitive relapse and refractory relapse (3). Patients with TFI <60 days are reportedly refractory to second-line chemotherapy (4). Topotecan is the standard therapy for patients with sensitive relapse SCLC (5,6). These patients may respond to the same induction chemotherapy (re-challenge chemotherapy) (7,8), with several studies reporting the efficacy of re-challenge chemotherapy (9,10). According to the NCCN guideline, re-challenge therapy is recommended for patients with TFI >180 days. However, it is not a standard therapy because of the lack of prospective large randomized trials in which a platinum plus etoposide was used. Meanwhile, chemotherapy regimens for SCLC patients with interstitial lung disease (ILD) are limited because amrubicin and irinotecan, which are both treatments for SCLC, can lead to pulmonary toxicity. Although re-challenge therapy can be effective in patients with sensitive relapse SCLC with ILD, its safety and efficacy are uncertain. Thus, this retrospective study aimed to investigate both the efficacy and safety of re-challenge chemotherapy in patients with sensitive relapse SCLC with ILD.
Methods
Patients and study design
We retrospectively reviewed the medical records of patients with sensitive relapse SCLC with pre-existing ILD treated with re-challenge therapy at our institute between July 2009 and July 2018. TFI was defined as the period from the date of completion of first-line chemotherapy to the first relapse. Sensitive relapse was defined as TFI of more than 60 days, and patients with sensitive relapse were re-challenged with platinum (carboplatin or cisplatin) plus etoposide chemotherapy including one patient who underwent treatment change from cisplatin to carboplatin at the time of re-challenge. Patients with idiopathic ILD was classified into two groups: the idiopathic pulmonary fibrosis (IPF) pattern group and the non-IPF pattern group. IPF pattern was diagnosed histologically or radiologically, and those with other patterns were categorized as non-IPF pattern. IPF was diagnosed according to the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association criteria: (I) exclusion of other known causes of ILD; (II) the finding of a usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography (HRCT) in patients who did not undergo surgical lung biopsy; and (III) specific combinations of HRCT and surgical lung biopsy pattern in patients who underwent surgical lung biopsy (11). Typical CT findings of IPF were basal predominant, subpleural reticular abnormality with honeycomb cysts and traction bronchiectasis without findings of atypical features of IPF such as consolidation, isolated cysts, and peribronchovascular nodules (12,13). Acute exacerbation of ILD was defined as meeting all the following criteria: (I) previous or concurrent diagnosis of ILD; (II) acute worsening or development of dyspnea typically within 1 month; (III) computed tomography with new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with UIP pattern; and (IV) deterioration not completely explained by cardiac failure or fluid overload (14). This study was approved by the Medical Research Ethics Committee of Osaka Habikino Medical Center. The final observation date was July 30, 2018. Performance status was assessed according to the Eastern Cooperative Oncology Group guidelines (15). Therapeutic effects were assessed using the Response Evaluation Criteria in Solid Tumours version 1.1 (16). Toxicities were graded according to the Common Terminology Criteria for Adverse Events version 4.0 (17).
Outcome parameters and statistical analyses
The outcome parameters measured in this study were progression-free survival (PFS) and overall survival (OS). PFS was calculated as the duration between the start of treatment and progression or death. OS was measured from the date of diagnosis or the date of starting re-challenge chemotherapy to the date of death. PFS and OS were assessed by using Kaplan-Meier analysis, and all statistical analyses were performed using R software (version 2.13.1; EZR Development Core Team 2011, R: a language and environment for statistical computing, Foundation for Statistical Computing; Vienna, Austria).
Results
Patients
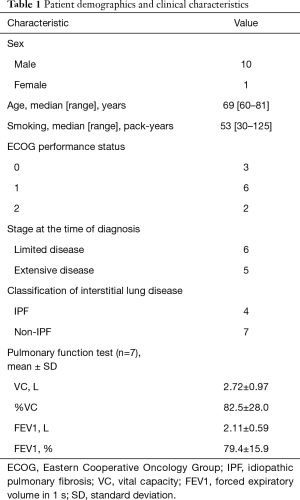
The clinical and demographic characteristics of the patients are shown in Table 1. Eleven patients (10 men and 1 woman) were treated with platinum plus etoposide re-challenge therapy during the study period. The median patient age was 69 years (range, 60–81 years). At the time of diagnosis, 6 and 5 patients had limited and extensive disease, respectively. Among the cases of interstitial pneumonia, 4 were classified as the IPF pattern group and 7 were classified as the non-IPF pattern group. The mean serum KL-6 (n=8) and albumin levels were 694 U/mL and 4.1 g/dL, respectively. In the respiratory function test, the percent of predicted vital capacity (%VC) was 82.5%±28.0%. Respiratory function test results were not obtained for 4 patients. All non-IPF patterns were chronic ILD. We could not identify the non-IPF type because no patients had a histological confirmation of interstitial pneumonias. One patient was diagnosed with IPF 5 years before the first-line chemotherapy, and the other patients were diagnosed with ILD and SCLC at the same time. No patients received treatments for ILD. One patient received whole-brain radiation therapy (WBRT) for prophylaxis of brain metastases and 5 patients received WBRT for treating brain metastases. One patient received palliative radiation for thoracic spine metastasis. All patients did not receive radical radiotherapy to the chest because they had ILD.
Full table
Response
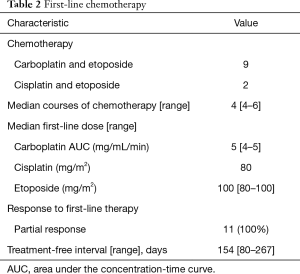
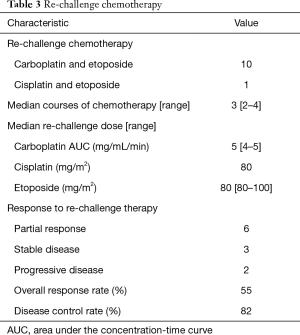
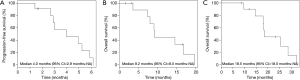
Regarding first-line treatment, 9 patients received carboplatin and etoposide, and 2 patients received cisplatin and etoposide (Table 2). A median of 4 cycles was administered. All patients achieved a partial response. The median TFI was 154 days (range, 80–267 days). In the re-challenge chemotherapy, 10 patients received carboplatin and etoposide, and 1 patient received cisplatin and etoposide. At the time of re-challenge chemotherapy, the doses for 3 cases were reduced because of haematological adverse event, and the regimen of 1 case was changed from cisplatin to carboplatin. The median re-challenge dose of carboplatin, cisplatin, and etoposide were area under the concentration-time curve 5 (mg/mL/min), 80 (mg/m2), and 80 (mg/m2), respectively. A median of 3 cycles was administered. Six patients had partial response, 3 had stable disease, and 2 patients had progressive disease (Table 3). The median PFS from the time of re-challenge was 4 months (95% CI, 2.9–NA). The median OS from the time of re-challenge and median OS from the time of diagnosis were 9.2 months (95% CI, 8.0–NA) and 18.5 months (95% CI, 18.0–NA), respectively (Figure 1). Six patients (55%) received further-line chemotherapy. Five patients received topotecan; of them, 1 patient had partial response, 3 had stable disease, and 1 had progressive disease. One patient received nanoparticle albumin-bound paclitaxel, and he had partial response.
Full table
Full table
Safety
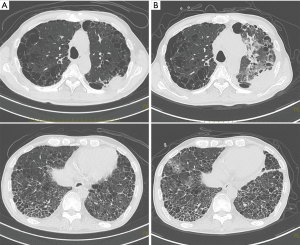
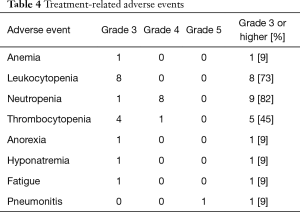
One patient who was identified with a UIP pattern developed acute exacerbation of ILD and disease progression 173 days after the last course of re-challenge (Figure 2). He died 8 days after acute exacerbation of ILD. The other patients died due to disease progression. Treatment-related adverse events are shown in Table 4. Although the most common hematological grade 3 or 4 adverse events was neutropenia (82%), no patient developed febrile neutropenia.
Full table
Discussion
The present study showed that platinum plus etoposide re-challenge therapy can be effective and should be considered in patients with sensitive relapse SCLC with pre-existing ILD. With respect to efficacy, in our study, the overall response rate was 55%. Median PFS and median OS from the time of re-challenge were 4 and 9.2 months respectively. Genestreti et al. reported an overall response rate of 45%, and a median PFS and median OS from the time of re-challenge of 5.5 and 7.9 months, respectively (9).
OS from time to re-challenge therapy is relatively long in our study. Ardizzoni et al. (4) reported that patients with liver metastasis and/or low albumin and/or PS2 have poor OS, and in our cases, no patients had liver metastasis and low albumin levels at the beginning of first-line chemotherapy and the re-challenge therapy. Subgroup analysis of patients who received chemotherapy alone as the first-line regimen in a retrospective study showed significantly PFS in the re-challenge group than the non-re-challenge group (median, 5.4 vs. 3.6 months, P=0.0038) (10). Similarly, our patients did not receive chemoradiotherapy because they had ILD. Re-challenge chemotherapy can be effective in patients with sensitive relapse SCLC with pre-existing ILD. Five patients in the current study received topotecan after discontinuing re-challenge therapy, and the disease control rate was 80%. There is a possibility that topotecan is effective after platinum re-challenge therapy for SCLC patients with pre-existing ILD. With respect to safety, one patient developed acute exacerbation of ILD 173 days after the last course of re-challenge. In this case, we cannot deny that chemotherapy was the main cause of the acute exacerbation of ILD although it developed late. The incidence of acute exacerbation of ILD related to platinum agents plus etoposide ranges from only 2.0–5.9% (18,19), and the combination of platinum agents plus etoposide is considered relatively safe. Although the incidence of leukocytopenia and neutropenia was high as previously reported (19), no patient developed febrile neutropenia, and the adverse events are manageable. Therefore, re-challenge chemotherapy can be considered in patients with sensitive relapse SCLC with pre-existing ILD. The JCOG0605 clinical trial showed that the combination of cisplatin, etoposide, and irinotecan could become the standard treatment for selected patients with sensitive relapse SCLC (20). However, its applicability is limited in clinical practice because chemotherapy requires long-term hospitalization, and the rate of severe myelosuppression is high. Moreover, irinotecan is contraindicated for interstitial pneumonia. Although amrubicin has also been reported to be more effective in the treatment of sensitive relapse SCLC than re-challenge chemotherapy, it is also contraindicated for interstitial pneumonia (21). Therefore, re-challenge with first-line platinum plus etoposide chemotherapy can be useful for sensitive relapse SCLC with pre-existing ILD based on our study results. Our study had limitations. First, we retrospectively analyzed the data from a single institution, and our sample size was small. Second, patient selection was confined to Japanese patients. Third, the non-IPF type was not confirmed. Further prospective studies are warranted to establish the optimal regimen for sensitive relapse SCLC with pre-existing ILD.
In conclusion, treatment of SCLC with pre-existing ILD is limited. From our study, re-challenge chemotherapy can be effective and considered in patients with sensitive relapse SCLC with ILD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board (IRB) approval for this study was obtained from the Medical Research Ethics Committee of Osaka Habikino Medical Center (IRB number: 919). Informed consent was waived by the IRB because of the retrospective nature of this study.
References
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 1997;15:2090-6. [Crossref] [PubMed]
- Ardizzoni A, Tiseo M, Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer 2014;50:2211-8. [Crossref] [PubMed]
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [Crossref] [PubMed]
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012-9. [Crossref] [PubMed]
- Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409-11. [Crossref] [PubMed]
- Giaccone G, Ferrati P, Donadio M, et al. Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol 1987;23:1697-9. [Crossref] [PubMed]
- Genestreti G, Tiseo M, Kenmotsu H, et al. Outcomes of Platinum-Sensitive Small-Cell Lung Cancer Patients Treated With Platinum/Etoposide Rechallenge: A Multi-Institutional Retrospective Analysis. Clin Lung Cancer 2015;16:e223-8. [Crossref] [PubMed]
- Naito Y, Yamada K, Imamura Y, et al. Rechallenge treatment with a platinum-based regimen in patients with sensitive relapsed small-cell lung cancer. Med Oncol 2018;35:61. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Johkoh T, Müller NL, Cartier Y, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology 1999;211:555-60. [Crossref] [PubMed]
- Nishimura K, Kitaichi M, Izumi T, et al. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992;182:337-42. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- U.S. Department of Health and Human Services. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010).
- Yoshida T, Yoh K, Goto K, et al. Safety and efficacy of platinum agents plus etoposide for patients with small cell lung cancer with interstitial lung disease. Anticancer Res 2013;33:1175-9. [PubMed]
- Minegishi Y, Kuribayashi H, Kitamura K, et al. The feasibility study of Carboplatin plus Etoposide for advanced small cell lung cancer with idiopathic interstitial pneumonias. J Thorac Oncol 2011;6:801-7. [Crossref] [PubMed]
- Goto K, Ohe Y, Shibata T, et al. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2016;17:1147-57. [Crossref] [PubMed]
- Inoue A, Sugawara S, Maemondo M, et al. Randomized phase II trial comparing amrubicin with re-challenge of platinum doublet in patients with sensitive-relapsed small-cell lung cancer: North Japan Lung Cancer Study Group trial 0702. Lung Cancer 2015;89:61-5. [Crossref] [PubMed]


