
Initial clinical impact of inhaled nitric oxide therapy for refractory hypoxemia following type A acute aortic dissection surgery
Introduction
Acute type A aortic dissection (TAAD) is a severe life-threatening cardiovascular disease that requires emergent surgical intervention. Although preoperative recognition, perioperative management, and surgical techniques have been significantly improved, TAAD operations are still associated with high mortality and morbidity (1,2). Hypoxemia is a frequent postoperative surgical complication in TAAD and is associated with increased mechanical ventilation duration, intensive care unit (ICU) stay, mortality and hospital costs (3). Although studies have raised questions about the association between TAAD and the risk factors for postoperative hypoxemia (4-6), the mechanism of hypoxemia in TAAD remains unclear but may be related to multifactorial causes including postoperative systemic inflammatory response syndrome, hypothermia, dissection, etc. Additionally, some TAAD patients with hypoxemia might be refractory to conventional treatment.
Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator administered as a gas through the airway. The benefits of iNO therapy are related to oxygenation improvement, which results from diverting pulmonary blood flow toward well-ventilated lung areas and improving ventilation-perfusion mismatch (7). iNO has been reported as an intervention to treat refractory hypoxemia in acute respiratory distress syndrome (ARDS) patients (7-11) and can improve systemic oxygenation (PaO2/FiO2).
Accordingly, we hypothesized that iNO might be beneficial for oxygenation in patients with postoperative refractory hypoxemia after surgery for TAAD. In the present study, we investigated the effects of iNO therapy on clinical outcomes in TAAD patients with refractory hypoxemia.
Methods
Study design
The study was conducted in a 39-bed cardiac surgery ICU (CSICU) at Zhongshan Hospital, which is affiliated with Fudan University in Shanghai, mainland China, from January 2016 to October 2017. This hospital, which is one of the largest cardiovascular surgical centers in mainland China, performs over 150 TAAD operations per year, including ascending aortic and hemi- or total-arch replacement concomitant with or without surgical treatment of the aortic root as well as a stent elephant trunk (MicroPort® CRONUS) procedure for the descending aorta.
All patients were routinely assessed by transthoracic echocardiography (TTE) preoperatively. During the operation, transesophageal echocardiography (TEE) was also routinely used to monitor hemodynamics and confirm the effect of surgery. Furthermore, TTE and lung ultrasound were used to identify different causes of hypoxemia during the postoperative period, such as right to left shunting, cardiac tamponade, obstructive shock, decreased ventricular systolic function, pneumothorax (PTX), pulmonary edema, pulmonary consolidation, pleural effusion or equivocal findings of pulmonary embolism (PE). For the patients with suspected PE, bedside cardiac ultrasonography and a venous examination of the proximal bilateral lower extremities were performed. An additional TEE examination was also considered if necessary.
Postoperative hypoxemia in TAAD patients was defined as a PaO2/FiO2 ratio of 200 mmHg or lower (5,6). Postoperative refractory hypoxemia was defined as a persistent PaO2/FiO2 ratio ≤100 mmHg despite conventional therapy within 6 hours of admission to the CSICU. In general, conventional treatment was carried out depending on the differential diagnoses for hypoxemia. Since December 2016, on the basis of conventional therapy, we explored the potential use of iNO to treat refractory hypoxemia. A retrospective study with historical control subjects was conducted to compare the clinical outcomes of iNO therapy in postoperative TAAD patients with refractory hypoxemia. Depending on the therapeutic intervention, patients with refractory hypoxemia were divided into two groups: the (I) conventional therapy group (January to November 2016) and the (II) iNO therapy group (December 2016 to October 2017). Data were collected from the medical records from the Zhongshan Hospital Electronic Health Record System. This study was approved by the Ethics Committee of Zhongshan Hospital Affiliated to Fudan University (No. B2016-141R) and was conducted in compliance with the institutional requirements. In the iNO therapy group, written informed consent was obtained from patients’ surrogates. A flowchart of patient enrollment is summarized in Figure 1.
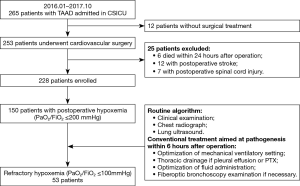
The exclusion criteria were as follows: (I) patients who did not receive cardiac surgery, such as those with no indications for surgical intervention and those who died before the surgical intervention; (II) patients who died within 24 hours of the operation; and (III) patients who developed postoperative complications, such as stroke or spinal cord injury, which may have led to respiratory failure.
Conventional therapy
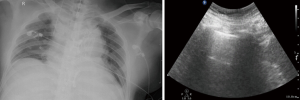
All patients with postoperative hypoxemia were routinely evaluated by clinical examination, and the examination included chest radiographs and lung ultrasound (Figure 2). A routine algorithm was implemented to diagnose the pathogenesis of hypoxemia, such as PTX, pulmonary edema, pulmonary consolidation, pleural effusion or airway obstruction. Accordingly, aimed at these causes, the conventional treatment included: (I) optimization of mechanical ventilation settings, including tidal volume adjustment and positive end expiratory pressure (PEEP) titration; (II) thoracic drainage if pleural effusion or PTX was present; (III) optimization of fluid management; and (IV) fiberoptic bronchoscopy examination if necessary.
iNO therapy and administration
Despite conventional management, certain patients still presented with refractory hypoxemia that was defined as a persistent PaO2/FiO2 ratio ≤100 mmHg within 6 hours of admission to the CSICU. These patients who were not responsive to conventional therapy received 5 ppm iNO therapy.
iNO was delivered through a gas flow-rate control system (NOD-200, LIHUA Science and Technology Co., Ltd., Hangzhou, China) using a bleed-in adapter placed in the inspiratory limb of the circuit, just distal to the humidifier. NO was supplied from tanks containing 1,000 ppm, with nitrogen used as the balance gas (FULIAN Gas Company, Suzhou, China). A high-purity, corrosion-resistant regulator with a very low flowmeter (25 to 200 mL/min, PARKER Veriflo, USA) was used to regulate the flow rate. NO and NO2 concentrations were continuously measured with an electrochemical sensor (Exidyne Instrumentation Technologies, Exton, PA, USA). A low dose of 5 ppm NO was administered to patients in this study (12).
iNO was weaned if the PaO2/FiO2 ratio was as high as 150 mmHg after a total time of 2 to 6 hours from iNO withdrawal. In this study, iNO was administered to patients who received mechanical ventilation during inspiration, and patients who were successfully extubated could still receive iNO through a noninvasive ventilation (NIV) mask or nasal cannulae if necessary (7). Patients were discharged from the CSICU after weaning from mechanical ventilation and iNO.
Criteria for extubation
For all patients, standard mechanical ventilation practices were carried out, such as a daily spontaneous breathing trial (SBT), chlorhexidine mouth hygiene, head of the bed elevation, and daily sedation interruptions. The SBT was performed with continuous positive airway pressure or a pressure support model, with a pressure support of 5 cmH2O and a PEEP of 5 cmH2O, lasting 30 to 60 minutes. The extubation criteria were described in our previous study: clear consciousness, stable hemodynamics, adequate oxygenation, and successful SBT (13). During the SBT, the patient was monitored for evidence of weaning failure. Patients were extubated if none of the following signs were observed: breathing frequency >35 breaths/min, SpO2 <90%, rapid shallow breathing index (respiratory rate/tidal volume) >105, 20% increase or decrease from the baseline heart rate or blood pressure, abdominal paradoxical movement, substantial agitation, anxiety, and/or diaphoresis.
Clinical outcomes
The primary endpoint of the study was the effect of iNO therapy on oxygenation (PaO2/FiO2), with special regard to changes over time. Secondary endpoints included the duration of invasive mechanical ventilation support, NIV support requirements, ICU mortality, length of ICU and hospital stays and postoperative complications [such as infections, incidence of acute kidney injury (AKI) and renal replacement therapy].
Statistical analysis
Categorical variables are described using frequencies and percentages, whereas continuous variables are described using the mean ± standard deviation. Patient demographics and clinical characteristics and the outcomes of patients who received iNO versus those who did not were compared using the chi-squared test or Fisher’s exact test (when the count in any cell of a contingency table was less than required) for categorical variables and a two-sample t-test to compare the means of normally distributed data for continuous variables. All tests were two-tailed, and a P value less than 0.05 was considered statistically significant. SPSS for Windows 19.0 (Chicago, IL, USA) was used for the statistical analysis.
Results
Patients
From January 2016 to October 2017, 265 patients with TAAD were admitted to the CSICU. Twelve patients developed aortic rupture, preoperative cardiac arrest or multiorgan malperfusion and did not receive surgical intervention. Six patients died within 24 hours postoperatively. The causes of death included uncontrolled bleeding in four patients, low cardiac output in one patient, and multiorgan malperfusion syndrome in one patient. Twelve patients developed stroke, and seven patients developed spinal cord injury, all of which required prolonged mechanical ventilation. In the remaining 228 patients undergoing surgical reconstruction for TAAD, 150 (65.79%) patients developed postoperative hypoxemia (PaO2/FiO2 ratio ≤200 mmHg). After comprehensive evaluation and interventions, 53 (23.25%) patients developed refractory hypoxemia (PaO2/FiO2 ratio ≤100 mmHg). Based on grouping, 26 patients received iNO therapy (iNO therapy group), and the remaining 27 patients received conventional therapy (conventional group) (Figure 1).
Basic characteristics and preoperative, intraoperative, and postoperative parameters
No significant differences in age, sex, body mass index (BMI), past medical history, comorbidities, aortic dissection complications (including pericardial effusion, ischemic stroke, lower limb ischemia, aortic regurgitation and mesenteric ischemia), preoperative laboratory examination findings, oxygenation (PaO2/FiO2), frequency of tracheal intubation and the time from onset to operation were found between the two groups (Table 1). The patients in the conventional group had a higher preoperative left ventricular ejection fraction (LVEF) (65%±4% vs. 63%±4%, P=0.036, Table 1) than the patients in the iNO therapy group.

Full table
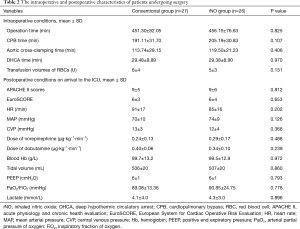
The intra- and postoperative data are summarized in Table 2. There was no statistically significant difference in terms of the operative time, cardiopulmonary bypass (CPB) time, clamping time and deep hypothermic circulatory arrest time. When patients were admitted to the CSICU, their acute physiology and chronic health evaluation (APACHE) II scores, European System for Cardiac Operative Risk Evaluation (EuroSCORE), hemodynamic parameters, ventilator parameters, vasoactive drug infusion rates, and hemoglobin and lactate levels were comparable (Table 2).
Full table
Improvement in oxygenation
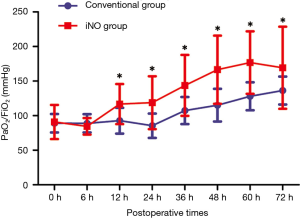
Oxygenation throughout the first 6 hours of admission to the CSICU did not significantly change in the conventional group (89.08±13.36 vs. 88.93±13.22 mmHg, P=0.457) or in the iNO therapy group (90.85±24.75 vs. 84.54±11.99 mmHg, P=0.314). The comparison between the two groups revealed that oxygenation was also comparable at the time of admission (90.85±24.75 vs. 89.08±13.36 mmHg, P=0.778) and 6 hours after conventional therapy (84.54±11.99 vs. 88.93±13.22 mmHg, P=0.645). With continuous NO inhalation, a higher PaO2/FiO2 ratio was observed in the iNO therapy group than in the conventional therapy group at 12, 24, 36, 48, 60 and 72 hours after admission to the CSICU (116.70±28.99 vs. 92.75±18.66 mmHg, P=0.003; 118.79±38.20 vs. 85.43±17.66 mmHg, P=0.001; 143.71±44.10 vs. 107.46±19.56 mmHg, P=0.002; 166.46±49.21 vs. 115.19±23.63 mmHg, P=0.000; 176.70±45.21 vs. 128.01±20.25 mmHg, P=0.000; and 169.31±59.44 vs. 136.38±20.26 mmHg, P=0.021, respectively) (Figure 3). The mean duration of iNO treatment was 79.96±31.83 hours.
Clinical outcomes
There was no statistically significant difference in mortality between the groups: the mortality rate was 7.41% (2/27 patients) in the conventional therapy group and 3.85% (1/26 patients) in the iNO therapy group. The duration of invasive mechanical ventilation in the iNO therapy group was significantly decreased compared with that in the conventional therapy group (69.19 vs. 104.56 hours, P=0.003). There were no significantly different outcomes with respect to the overall duration of ICU stay (9.88±3.08 vs. 12.36±5.22 days, P=0.059), hospital stay (16.88±3.33 vs. 20.76±9.36 days, P=0.060), or postoperative complications [such as infections, occurrence of AKI or renal replacement therapy] (14) (Table 3).

Full table
Discussion
In this study, the results indicated that iNO therapy in patients with refractory hypoxemia after surgery for TAAD was associated with improved arterial oxygenation and a reduced duration of invasive mechanical ventilation.
Hypoxemia is a common complication following cardiothoracic surgery (15). The incidence of hypoxemia following cardiothoracic surgery is approximately 7% (16). The incidence increases to 50.88% in patients who undergo surgery for TAAD (5). Furthermore, prolonged mechanical ventilation is generally required in patients with postoperative hypoxemia (17). According to previous studies, hypoxemia following surgery for TAAD is associated with several risk factors, including a long duration of CPB, aortic root occlusion, deep circulatory arrest, various types of pulmonary injuries and an excessive inflammatory response (15,17). In this study, although we tried to optimize the settings of mechanical ventilation, 53 patients (53/228, 23.25%) were unresponsive to conventional therapies and developed refractory hypoxemia with no elucidated pathogenesis. These patients could not be diagnosed with ARDS because they did not have typical radiological pulmonary infiltrates. Furthermore, hypoxemia could not be relieved by higher PEEP. Few studies have investigated the interventions used to treat refractory hypoxemia in such patients. To the best of our knowledge, this study is the first to analyze the effects of iNO therapy on refractory hypoxemia in patients following surgery for TAAD.
Early in the 1990s, iNO emerged as a potential therapy for ARDS because it decreased pulmonary vascular resistance without affecting systemic blood pressure and improved oxygenation by redistributing pulmonary blood flow toward ventilated lung units in patients with this condition (11). Subsequently, numerous investigations have shown the value of improving oxygenation in acute lung injury (ALI)/ARDS patients (12,18,19) while failing to demonstrate improvement in mortality (19-22). These prior studies inspired us to explore whether iNO therapy might have a potential effect on refractory hypoxemia following surgery for TAAD. In the study, we found that iNO therapy did produce a sustained improvement in oxygenation in patients with refractory hypoxemia after surgery for TAAD within 72 hours. Patients treated with iNO had a significantly higher PaO2/FiO2 ratio than those in the conventional therapy group (Figure 3). Thus far, the mechanisms underlying the association between hypoxia and surgery for TAAD are unclear. In the population of patients with refractory hypoxemia in our study, we ruled out common pathological changes associated with hypoxemia, such as sputum obstruction, interstitial pulmonary edema or extrapulmonary changes, through systemic assessments, including clinical observations, radiographs and lung ultrasound. Inferentially, the underlying mechanism for refractory hypoxemia after TAAD may be attributed to an intrapulmonary shunt due to an excessive inflammatory response. Furthermore, the clinical effect of iNO therapy on patients with refractory hypoxemia in this study might indirectly support our hypothesis.
Additionally, we sought to determine whether improved oxygenation would translate into a clinically relevant endpoint (i.e., the duration of invasive mechanical ventilation and ICU stay). Due to the improvement in oxygenation, the duration of invasive mechanical ventilation was significantly reduced with iNO therapy compared with conventional therapy because iNO could be administered to patients even after they were extubated and removed from the ventilator. Regardless, iNO therapy did not improve the outcome of mortality in this study. Similar results were also found in patients with acute hypoxemic respiratory failure in a meta-analysis (23).
Due to a lack of evidence concerning any beneficial effect of iNO therapy on clinical outcomes (19-22), the therapeutic applications of iNO in patients with ALI or ARDS remain uncertain. Currently, the Food and Drug Administration (FDA) has approved inhaled NO only for use in the pediatric population for hypoxic respiratory failure in term or near-term newborns (7,24). Previously described or merely postulated side effects of iNO therapy, such as NO2 toxicity (25), increased levels of methemoglobin (26), increased risk of renal dysfunction (20,27,28), increased bleeding times and inhibited platelet aggregation (29,30), were found to be fatal after a massive overdose of iNO (500 to 1,000 ppm) (31). Based on previous studies on the safety of iNO therapy, all of these side effects were associated with high concentrations of iNO (27-29,32-34). It should be mentioned that we did not administer higher doses of iNO in this study. The clinical efficacy was satisfied at low doses of 5 ppm, and no adverse events were found.
This study has some potential limitations. First, this was a single center, historically controlled study. The retrospective data collection and outcome evaluation might have caused a certain inevitable bias. Second, the study population was quite small and limited; we did not perform calculations to determine the required sample size before the study. Therefore, the findings merit future study with prospective trials; a larger sample size in a multicenter randomized controlled clinical trial would be expected to add more weight to these results. Third, several points regarding the effect of iNO therapy are lacking and need further investigation, such as dose titration of iNO and the optimal duration of iNO treatment. Fourth, our study also lacked invasive and noninvasive mechanical ventilator data, such as the mean airway pressure, plateau pressure, total pulmonary compliance changes, and noninvasive mechanical ventilation support duration, which could have potentially affected the outcomes observed in the study.
Conclusions
This retrospective analysis suggests that the addition of iNO may be considered a therapy for patients with refractory hypoxemia after surgery for TAAD. This approach may lead to sustained improvement in oxygenation and a reduced duration of invasive mechanical ventilation. Future research to optimize iNO implementation during clinical practice and to further elucidate the pathogenesis of hypoxemia following surgery for TAAD is warranted.
Acknowledgements
Funding: This article was supported by grants from the National Natural Science Foundation of China (grant No. 81500067), the Natural Science Foundation of Shanghai (grant No. 16ZR1405600) and the research funds of Zhongshan Hospital (grant No. 2017ZSYXQN23, 2016ZSQN23 and 2017ZSQN16).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Zhongshan Hospital Affiliated to Fudan University (No. B2016-141R) and was conducted in compliance with the institutional requirements. In the iNO therapy group, written informed consent was obtained from patients’ surrogates.
References
- Charlton-Ouw KM, Azizzadeh A, Sandhu HK, et al. Management of common carotid artery dissection due to extension from acute type A (DeBakey I) aortic dissection. J Vasc Surg 2013;58:910-6. [Crossref] [PubMed]
- Olsson C, Hillebrant CG, Liska J, et al. Mortality and reoperations in survivors operated on for acute type A aortic dissection and implications for catheter-based or hybrid interventions. J Vasc Surg 2013;58:333-339.e1. [Crossref] [PubMed]
- Yang Y, Sun L, Liu N, et al. Effects of Noninvasive Positive-Pressure Ventilation with Different Interfaces in Patients with Hypoxemia after Surgery for Stanford Type A Aortic Dissection. Med Sci Monit 2015;21:2294-304. [Crossref] [PubMed]
- Liu N, Zhang W, Ma W, et al. Risk factors for hypoxemia following surgical repair of acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2017;24:251-6. [PubMed]
- Nakajima T, Kawazoe K, Izumoto H, et al. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today 2006;36:680-5. [Crossref] [PubMed]
- Sheng W, Yang HQ, Chi YF, et al. Independent risk factors for hypoxemia after surgery for acute aortic dissection. Saudi Med J 2015;36:940-6. [Crossref] [PubMed]
- Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med 2005;353:2683-95. [Crossref] [PubMed]
- Bhatraju P, Crawford J, Hall M, et al. Inhaled nitric oxide: Current clinical concepts. Nitric Oxide 2015;50:114-28. [Crossref] [PubMed]
- Ferguson ND, Guérin C. Adjunct and rescue therapies for refractory hypoxemia: prone position, inhaled nitric oxide, high frequency oscillation, extra corporeal life support. Intensive Care Med 2018;44:1528-31. [Crossref] [PubMed]
- Krafft P, Fridrich P, Fitzgerald RD, et al. Effectiveness of nitric oxide inhalation in septic ARDS. Chest 1996;109:486-93. [Crossref] [PubMed]
- Rossaint R, Falke KJ, Lopez F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 1993;328:399-405. [Crossref] [PubMed]
- Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 2004;291:1603-9. [Crossref] [PubMed]
- Hao GW, Ma GG, Liu BF, et al. Evaluation of two intensive care models in relation to successful extubation after cardiac surgery. Med Intensiva 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [PubMed]
- Huffmyer JL, Groves DS. Pulmonary complications of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 2015;29:163-75. [Crossref] [PubMed]
- Mack MJ, Brown PP, Kugelmass AD, et al. Current status and outcomes of coronary revascularization 1999 to 2002: 148,396 surgical and percutaneous procedures. Ann Thorac Surg 2004;77:761-6. [Crossref] [PubMed]
- Weissman C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth 2004;8:185-211. [Crossref] [PubMed]
- Dellinger RP, Trzeciak SW, Criner GJ, et al. Association between inhaled nitric oxide treatment and long-term pulmonary function in survivors of acute respiratory distress syndrome. Crit Care 2012;16:R36. [Crossref] [PubMed]
- Lundin S, Mang H, Smithies M, et al. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med 1999;25:911-9. [Crossref] [PubMed]
- Adhikari NK, Burns KE, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 2007;334:779. [Crossref] [PubMed]
- Michael JR, Barton RG, Saffle JR, et al. Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med 1998;157:1372-80. [Crossref] [PubMed]
- Troncy E, Collet JP, Shapiro S, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med 1998;157:1483-8. [Crossref] [PubMed]
- Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxemic respiratory failure in children and adults. Cochrane Database Syst Rev 2003.CD002787. [PubMed]
- American Academy of Pediatrics. Committee on Fetus and Newborn. Use of inhaled nitric oxide. Pediatrics 2000;106:344-5. [Crossref] [PubMed]
- Sokol GM, Van Meurs KP, Wright LL, et al. Nitrogen dioxide formation during inhaled nitric oxide therapy. Clin Chem 1999;45:382-7. [PubMed]
- Weinberger B, Laskin DL, Heck DE, et al. The toxicology of inhaled nitric oxide. Toxicol Sci 2001;59:5-16. [Crossref] [PubMed]
- Ruan SY, Huang TM, Wu HY, et al. Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials. Crit Care 2015;19:137. [Crossref] [PubMed]
- Ruan SY, Wu HY, Lin HH, et al. Inhaled nitric oxide and the risk of renal dysfunction in patients with acute respiratory distress syndrome: a propensity-matched cohort study. Crit Care 2016;20:389. [Crossref] [PubMed]
- Högman M, Frostell C, Arnberg H, et al. Bleeding time prolongation and NO inhalation. Lancet 1993;341:1664-5. [Crossref] [PubMed]
- Samama CM, Diaby M, Fellahi JL, et al. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 1995;83:56-65. [Crossref] [PubMed]
- Greenbaum R, Bay J, Hargreaves MD, et al. Effects of higher oxides of nitrogen on the anaesthetized dog. Br J Anaesth 1967;39:393-404. [Crossref] [PubMed]
- George TN, Johnson KJ, Bates JN, et al. The effect of inhaled nitric oxide therapy on bleeding time and platelet aggregation in neonates. J Pediatr 1998;132:731-4. [Crossref] [PubMed]
- Germann P, Braschi A, Della Rocca G, et al. Inhaled nitric oxide therapy in adults: European expert recommendations. Intensive Care Med 2005;31:1029-41. [Crossref] [PubMed]
- Hugod C. Effect of exposure to 43 ppm nitric oxide and 3.6 ppm nitrogen dioxide on rabbit lung. A light and electron microscopic study. Int Arch Occup Environ Health 1979;42:159-67. [Crossref] [PubMed]


