
Editorial on comparison between stereotactic body radiotherapy and surgery in early stage NCSLC by Scotti et al.
Background
Lung cancer is the increasing trend in the world with the exception of some of the countries such as the United States and the UK and is the number one cause of cancer death. Recently, early stage non-small cell lung cancer and isolated lung tumor not larger than 3 cm in size have increasingly been discovered due to the widespread use of medical examination such as computed tomography (CT). The frequency of isolated lung tumor larger than 3 cm in size whose pathological diagnosis cannot be determined for a variety of reasons and which is diagnosed clinically as primary lung cancer has been increasing year by year with the increase in the elderly.
For clinical stage IA, “surgery of lobectomy or more with hilar and mediastinal lymph node dissection” is thought to be the standard treatment regardless of the size or the location of the primary tumor and limited surgery is positioned as investigational test treatment not the standard therapy (1). The report by Scotti et al. (2) also excluded cases receiving limited operation such as segmental resection or wedge resection. Even in the clinical stage IA, stereotactic body radiation therapy (SBRT), also referred to as stereotactic ablative body radiation (SABR) is the standard treatment for patients whose postoperative lung function is predicted to be insufficient or patients who cannot perform surgery including the limited surgery due to complications such as pulmonary fibrosis, emphysema, or cardiovascular disease (1). SBRT is recommended for the “surgery rejected” patient by reason of old age, high risk of anesthesia, concern about the decrease in postoperative lung function, or fear to the surgery even in operable patient who can perform surgery including limited surgery. In the National Comprehensive Cancer Network (NCCN) guideline version 2.2019, SBRT is an appropriate option for a group in which minimally invasive limited surgery is possible although normal lobectomy is impossible or for elderly persons of 75-year-old or older (1). SBRT has become a standard treatment for patients with medically inoperable early-stage lung cancer. However, its effectiveness in patients medically suitable for surgery is unclear.
Definition of inoperable patients
In the American College of Chest Physicians (ACCP) guideline, surgical indication criteria of lobectomy for impaired pulmonary function is defined as postoperative predictive forced expiratory volume in 1 second (%FEV1.0) of not less than 40% since surgery-related mortality increases if less than 40% (3). Additionally, performance status (PS) of 2–4 may be appropriate as the definition of inoperable since the criterion of PS 0 or 1 is used as eligibility criteria of resection in conventional clinical trials. The definition of inoperable cases is not confirmed and since it is various each study, it is necessary to pay attention to interpretation of the results. Scotti et al. (2) defined inoperable patient as (I) poor performance status, (II) major comorbidities (severe emphysema or other chronic obstructive pulmonary disease, myocardial infarction within the past 6–8 weeks, etc.), or (III) severe functional contraindications, such as prior pulmonary surgery, %FEV1.0 <1 L/min, cardiopulmonary test with a maximum oxygen consumption <10–15 mL/kg/min, shuffle walk test of <25 shuffles, or desaturation >4%.
Evaluation of residual tumor after SBRT
After SBRT, scarring of tumor tissue and inflammation of surrounding lung parenchyma occurs and these changes by scarring or inflammation continue for several months. Therefore, it is difficult to evaluate strictly whether the primary tumor to which SBRT was implemented has disappeared or is remaining. For that reason, even if it is judged that the remaining is not recognized in reference to imaging findings and clinical findings, it is not necessarily concluded that tumor cell has disappeared. Patients in the investigation by Scotti et al. (2) were followed using contrast-enhanced chest and abdomen CT every 3 months for the first 2 years; from the third year on, a total body CT scan was requested every 6 months, and suspicious CT progressions were then investigated with an 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT scan followed by biopsy if possible.
Determination of lung cancer
Three prospective studied from Europe and America about SBRT for clinical stage I lung cancer included 20–30% of patient without histological diagnosis, but no significant difference was recognized in local control or survival rate in comparison to patients with confirmed histological diagnosis by pathological diagnosis (4-6). Additionally, these studies suggest that the same local control and overall survival (OS) rate can be obtained even in the only diagnosis by imaging diagnosis including FDG-PET as in the case without histological diagnosis. Also, due to the further aging of the future, it is expected to increase lung tumor without histological diagnosis in inoperable or surgery refusal patients. In the study by Scotti et al. (2), histological diagnosis was undetermined in 22 out of SBRT 93 cases (24%). As a retrospective study, this rate is not higher than the above previous reports at all. Moreover, if histological diagnosis is not determined, benign tumor should be excluded as much as possible. In SBRT group of the research by Scotti et al. (2), patients treated without cytohistological confirmation of malignancy, PET positivity, and increasing dimensions on CT scans were considered to present diagnostic criteria of tumor presence. However, still a possibility that the patient with benign lung tumor is registered cannot be reduced to zero.
SBRT dose
In Europe and the United States, prescribed dose line is matched with marginal part of planning target volume (PTV) and planning is conducted so that prescribed dose becomes 60–90% of central dose. Regarding dose prescription, dose of PTV margin and mean dose are not ensured in the isocenter prescription and isocenter prescription is becoming less common internationally. The paper by Scotti et al. (2) describes that all biologically effective dose with an alpha/beta ratio of 10 values (BED10) of at least 100 Gy referred to the dose at the isocenter, with the 95% isodose encompassing the PTV. The impression is gotten that the radiation dose is relatively higher since 71% patients received more than 150 Gy in BED10. However, I wanted to know the concrete total irradiated dose and number of fractionation as well as BED10. It seems that in their institution (2), treatment planning for volumetric modulated arc therapy (VMAT) or tomotherapy that makes radiation dose within PTV relatively uniform (i.e. heterogeneity index is close to one) has been performed rather than create a hot-spot to PTV center. It is not written clearly in their report (2) whether the central lesions as well as peripheral lesions were included.
Postoperative adjuvant chemotherapy
For clinical stages IB (cT2aN0M0) and IIA, adjuvant chemotherapy in addition to surgery is conducted. It has been shown that the prognosis is improved by the administration of postoperative tegafur/uracil combination drug (UFT) for clinical stage IB (Hazard ratio 0.48, 95% CI: 0.29–0.81) (7). Additionally, it is recommended to perform chemotherapy including cisplatin for complete resection case with postoperative pathological stage II (8). There is no description of adjuvant therapy after surgery in the paper by Scotti et al. (2), although it included stages IB and IIA.
Local recurrence
It is common that the local recurrence after surgery is defined as the recurrence in resection stump and regional recurrence other than surroundings of the primary site (i.e. regional lymph node such as hilar, mediastinal, and supraclavicular). On the other hand, after SBRT, it is often defined as the recurrence just in surroundings of the primary site in a daily medical practice. This reason is the difference of treatment extent between surgery and SBRT. Namely, in standard surgery, lobectomy including regional lymph node dissection is conduced and in SBRT, only primary site is irradiated without irradiation of regional lymph node. In the comparison between surgery and SBRT, it is necessary to note this point. In the paper by Scotti et al. (2), locoregional control was defined as an absence of relapse within the tumor treatment site, ipsilateral hilum, and ipsilateral mediastinum. The previous prospective studies reported that 3-year local control rate was 86–98% (Table 1). In the study by Scotti et al. (2), median follow-up time was 23 months and local recurrence was 9 out of 93 cases (9.7%) in the SBRT group and 3 out of 94 cases (3.2%) in the lobectomy (LOB) group, respectively.
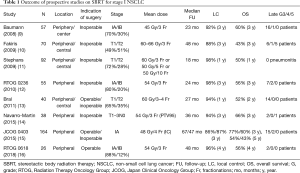
Full table
Survival
According to the national aggregate of 2004 in Japan, the frequency of each clinical stage (TNM classification 7th Edition) in resected case for non-small cell lung cancer (NSCLC) was that 54.0% in stage IA, 20.1% in stage IB, and 7.0% in stage IIA and 5-year OS rate was 82.0%, 66.1%, and 54.5%, respectively (17). The 3-year OS rate after SBRT for clinical stage I NSCLC including clinical stage IB is 56–76% and the result is better as compared with conventional methods and its usefulness is obvious (Table 1). The 3-year OS rate after SBRT of patients who were unable to perform standard surgery in JCOG 0403 was 59.9% (15). In the paper by Scotti et al. (2), stage IA and IB were included in 72% and 21%, respectively and it was not inferior to the above outcomes that 3-year OS rate was 73% in the LOB group and 65% in the SBRT group. Although Scotti et al. (2) argue that no difference in OS was observed between 30 operable patients undergoing SBRT and 94 LOB cases, clinical background factors of only operable patients undergoing SBRT were not specified. Especially the number of cases of operable patients undergoing SBRT was very small and there may be insufficient power to conclude that there is no difference in OS between the two groups. It may be better to perform the propensity score matching since the background such as performance status and age is disadvantageous for SBRT group, although it may be difficult due to the small number of cases.
Adverse event after SBRT
Zhang et al. (18) performed meta-analysis on the optimal dose using BED10 for clinical stage I NSCLC and compared in four groups of low (<83.2 Gy), medium (83.2–106 Gy), medium to high (106–146 Gy), and high (>146 Gy) and the 5-year OS rate was better in medium and medium to high group than in low and high group. It was pointed out as this reason that survival rate was reduced because of increase of adverse events in high (>146 Gy) group. Grade 3 or more adverse events was 12.7% and 16% in RTOG 0236 and RTOG 0618, respectively, which adopted 54 Gy in three fractionations (BED10 =151.2 Gy). Grade 3 or higher adverse events occurrence rate was 9.6% in inoperable patients of JCOG 0403 (15). In the paper by Scotti et al. (2), there was not the description of side effects.
Randomized clinical trial
Recent pooled analysis of two incomplete STARS (NCT00840749) and ROSEL (NCT00687986) randomized trials comparing SABR versus lobectomy have shown a significantly improved 3-year survival with SABR of a 15% advantage in enrolled 58 patients (19). Grade 3/4/5 treatment-related adverse events were seen in 3/0/0 patents in the SABR group and 10/1/1 patients in the surgery group, respectively.
Two phase III trials from the United States comparing SBRT and surgery in stage I NSCLC are continuing and are expected to bring noticeable information about the optimal treatment strategy. The first one is the VALOR trial (NCT02984761). Allocation is randomized, estimated enrollment is 670 participants, and estimated primary completion date is September 30, 2026. Any patient has a preliminary diagnosis of stage I NSCLC and primary tumor size is less than or equal to 5 cm by CT. Peripheral tumors will receive any one of 54 Gy in 3, 56 Gy in 4, 57.5 Gy in 5 fractions, while central tumors will be treated with 50 Gy in 5 fractions. Surgery will undergo a standard lobectomy or limited anatomic pulmonary resection (segmentectomy), but non-anatomic (wedge) resections are not permitted. Participants found to have incidental nodal involvement after surgery will be referred for adjuvant chemotherapy, without postoperative radiotherapy. The second one is the “Stablemates” trial (NCT02468024). Estimated enrollment is 272 participants, allocation is randomized, and estimated primary completion date is December 2020. SBRT adopts 54 Gy in 3 fractions for biopsy confirmed NSCLC located peripherally within the lung and tumor ≤4 cm maximum diameter, including clinical stage IA and selected IB. The primary objective of this study is to test the hypothesis that the 3-year OS in high risk operable patients with Stage I NSCLC is greater in patients who undergo SBRT as compared to standard sublobar lung resection. The institutions in the U.S., Canada and Australia are taking part in this clinical trial. Patients will be accrued and followed for a minimum of 2-years after treatment.
Conclusions
The standard of care for stage I non-small cell lung cancer has historically been surgical resection in patients who are medically fit to tolerate an operation. Multiple retrospective studies have demonstrated that the outcomes with surgery are likely equal or superior. Recent data including the results from a pooled analysis of two incomplete phase III studies are promising and now suggests that stereotactic radiotherapy may be a suitable alternative.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- National Comprehensive Cancer Network (NCCN) Guidelines. Non-small-cell lung cancer version 2. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Scotti V, Bruni A, Francolini G, et al. Stereotactic Ablative Radiotherapy as an Alternative to Lobectomy in Patients With Medically Operable Stage I NSCLC: A Retrospective, Multicenter Analysis. Clin Lung Cancer 2019;20:e53-61. [Crossref] [PubMed]
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Taremi M, Hope A, Dahele M, et al. Stereotactic body radiotherapy for medically inoperable lung cancer: prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys 2012;82:967-73. [Crossref] [PubMed]
- Bradley JD, Naqa IE, Drzymala RE, et al. Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 2010;77:1146-50. [Crossref] [PubMed]
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: Comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011;101:250-4. [Crossref] [PubMed]
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil–tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [Crossref] [PubMed]
- Arriagada R, Auperin A, Burdett S, et al. NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non–small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009;4:838-44. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Bral S, Gevaert T, Linthout N, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys 2011;80:1343-9. [Crossref] [PubMed]
- Navarro-Martin A, Cacicedo J, Leaman O, et al. Comparative analysis of thermoplastic masks versus vacuum cushions in stereotactic body radiotherapy. Radiat Oncol 2015;10:176. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: findings from the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018;4:1263-6. [Crossref] [PubMed]
- Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. [Crossref] [PubMed]
- Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]


