Regional capnometry to evaluate the adequacy of tissue perfusion
Introduction
In clinical practice, it is difficult to find relevant hemodynamic and oxygenation parameters that can serve as titration endpoints for hemodynamic interventions. In patients with shock or having undergone major surgery associated with high incidence of postoperative complications, the accepted goal for hemodynamic optimization is to increase O2 delivery (DO2) and thus O2 consumption (VO2) (1). However, recent studies have failed to confirm that hemodynamic optimization reduces morbidity and mortality (2-4). Even when macrocirculatory targets are met, microcirculatory disturbances can persist and lead to organ dysfunction (5). Hence, improved hemodynamic management might only be achieved by detecting VO2’s responsiveness to an increase in DO2 (i.e., in patients with anaerobic metabolism) (6). To date, several variables have been described as markers of tissue perfusion: oxygen venous saturation (SvO2) (7), the veno-arterial PCO2 gradient (PCO2 gap) (8), the arterial lactate level, and the ratio of the veno-arterial PCO2 gradient to the arteriovenous content difference in O2 (i.e., the PCO2 gap/DavO2 ratio) (9-12). Although these variables have been studied in intensive care units (ICUs) and operating theaters, they do have some limitations (described in other chapter of this publication). Moreover, these conventional variables are markers of systemic hypoperfusion, and so are not able to detect regional hypoperfusion (5).
In the 1990s, Nakagawa et al. and Tang et al. suggested that an increase in tissue PCO2 (“stagnant hypercapnia”) was a marker of inadequate tissue perfusion (13,14). Indeed, the difference between regional tissue PCO2 and PaCO2 (the tissue-arterial PCO2 gap) is an earlier, more accurate marker of regional tissue hypoperfusion than whole-body parameters are (15,16). This concept has been validated in many animal models and clinical studies (13,17-20). From a physiological point of view, an increase in tissue PCO2 results from two mechanisms that must both be present to produce “stagnant hypercapnia”. Firstly, an increase in tissue CO2 which can results of rises in aerobic metabolism with greater CO2 generation by the cells or results of tissue hypoxia with an increase in anaerobic glycolysis and excessive production of lactic acid (17). Secondly, the maintenance of blood flow easily removes CO2 into the venous circulation via the “washout phenomenon” (21). Thus, stagnant hypercapnia can occur only when blood flow is abnormally low. Thus, PCO2 gap increases as cardiac output falls in case of tissue hypoxia or without tissue hypoxia (22,23) (Figure 1).
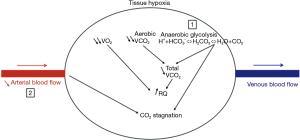
These findings have been confirmed by some clinical studies (18,24,25)—most notably by Vallet et al.’s study of limb-PCO2 gap (26). These researchers demonstrated that the PCO2 gap increased when the DO2 fell after a reduction in blood flow (ischemic hypoxia) but not when DO2 fell with maintenance of blood flow (hypoxic hypoxia). These results have been confirmed in animal studies of the tissue-arterial PCO2 gap (19,20); the latter increased during ischemic hypoxia but not during non-ischemic hypoxia.
Hence, these findings suggest that the tissue-arterial PCO2 gap is a marker of tissue hypoperfusion in general, and not just in cases of hypoxia. The normal reference range for the tissue PCO2 gap is 8 to 10 mmHg (20,27).
The objectives of the present review were to describe the sites at which regional PCO2 and tissue-arterial PCO2 gap have been measured (gastric, sublingual, transcutaneous and bladder sites), assess this parameter’s prognostic value, and evaluate its utility in goal-directed therapy.
Gastric intramucosal PCO2 (PgCO2)
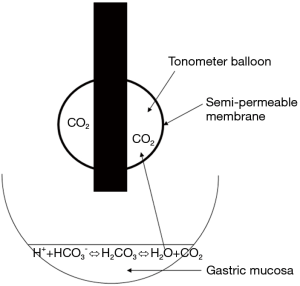
The tonometric measurement of regional CO2 pressures is based on equilibration of a gas’s partial pressure between two compartments separated by a semi-permeable membrane. Using air or saline as an equilibration medium enables the gas analyzer to automatically measure the PCO2 at a balloon located at the end of a gastric tube (Figure 2). The PCO2 in the collected air is measured using infrared spectrometry. The stomach is easy to access, and is known to be highly sensitive to tissue hypoperfusion (28). Furthermore, PgCO2 measurements have been used to detect early splanchnic ischemia (29).
In the event of tissue hypoxia and low VO2, CO2 production by the gastric mucosa increases. Thus, it has been suggested that PgCO2 is a marker of tissue hypoxia (30) and can predict morbidity and mortality in critically ill patients (31). However, as mentioned in the introduction, the PgCO2 − PaCO2 gradient (PgCO2 gap) might be more valuable because it reflects the adequacy of gastric mucosal blood flow. In critically ill patients, the PgCO2 gap values measured on admission to the ICU and 24 h later constituted an independent prognostic factor for 28-day mortality, with a cut-off of >20 mmHg (16). In a perioperative setting, this index was predictive of postoperative complications (27,32).
When used as a prognostic tool in critically ill patients, the PgCO2 gap decreases upon fluid challenge. The change is related to the baseline PgCO2: PCO2 gap responders were defined by a decrease of more than 3 mmHg. Even more interestingly, whole-body indexes of oxygenation (SvO2, VO2 and PCO2 gap) remained unchanged after fluid challenge, when the PgCO2 gap decreased (33).
The main limitations of this method are the need for concomitant H2-blocker use and the discontinuation of enteral feeding (34). Moreover, this type of device has not been developed commercially, thus limiting its availability.
Esophageal tonometry has been proposed as a convenient alternative to gastric tonometry in animal models of shock (35,36). Good inter-variable correlations were found at this measurement site. However, this site is more difficult to access, which explains its scarce use in clinical practice.
Sublingual PCO2 (PslCO2)
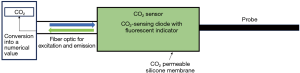
The most intensively developed and well-studied sublingual CO2 sensor is the CapnoProbe® CO2-sensing optode (Nellcor, Pleasanton, CA, USA) (31,37-40). The optode contains a CO2-sensing fluorescent dye that is excited by light conducted through an optical fiber. The emitted fluorescence is then transmitted back to the instrument (Figure 3) (31).
Interest in the sublingual region has been stimulated by orthogonal polarization spectral imaging studies that have evidenced a decrease in sublingual capillary density in the event of septic shock (41,42). A study performed in the 1980s found that PslCO2 was elevated in a model of hemorrhagic shock (43). In animal studies of hemorrhagic and septic shock, sublingual CO2 measurements are well correlated with PgCO2 and whole-body markers of tissue hypoperfusion (13,43,44). The main advantages of this technique relate to its noninvasive nature, the absence of a requirement for withdrawing enteral feeding, and the correlation with the splanchnic region.
The basal value of PslCO2 was found to be predictive of mortality in acute circulatory failure and was associated with arterial lactate levels. When PslCO2 exceeded a threshold of 70 mmHg, its positive predictive value was excellent. Conversely, PslCO2 fell more quickly than arterial lactate during resuscitation (45).
However, the most interesting marker appears to be the PslCO2 − PaCO2 gradient (the PslCO2 gap). It is reportedly a better prognostic factor than whole-body markers (SvO2, cardiac index, DO2, and arterial lactate), and the best cut-off value was 25 mmHg (15). The PslCO2 gap may serve as an index of tissue dysoxia and the severity of tissue hypoperfusion in critically ill patients (38,39).
With regard to the PslCO2 gap’s potential as a treatment target (endpoint), it has been showed that the reperfusion of the damaged sublingual microcirculation (assessing using orthogonal polarization spectral imaging) was associated with the normalization of the PslCO2 gap during the resuscitation of patients with septic shock (37). However, the PslCO2 gap’s potential value as an endpoint during resuscitation has not yet been evaluated in critically ill patients. Although, this technique has been tested at the bedside, the CapnoProbe® is no longer commercially available.
Transcutaneous PCO2 (PcCO2)
Transcutaneous measurement of tissue CO2 is a simple, noninvasive technique. Interest in this method has been stimulated by studies of capnometry at the earlobe (46). The system includes a Severinghaus heated PCO2 electrode and a pulse oximetry sensor clipped to the earlobe (TOSCA® 500 monitor, Linde Medical Sensors, Basel, Switzerland). In a study of patients in the ICU, the transcutaneous PCO2 (PcCO2) was well correlated with PaCO2 (47-49). Other devices based on the same technology (SenTec AG, Basel, Switzerland) have yielded the same accuracy (50). However, a recent study found that measurement repeatability was poor (51).
Another study focused on the gradient between PcCO2 and PaCO2 (the PcCO2 gap) (52). The baseline PcCO2 gap levels were significantly higher in patients with septic shock, and the decrease after resuscitation was significantly greater in survivors than in non-survivors. Interestingly, survivors and non-survivors did not differ with regard to the change over time in whole-body parameters (cardiac output and ScVO2). A PcCO2 gap above 16 mmHg on day one was associated with a poor outcome. Interestingly, the variations in the PcCO2 gap during fluid challenge were inversely correlated with changes in microcirculatory skin blood flow.
Even though a large number of studies have investigated transcutaneous PCO2 in the field of neonatology, a Cochrane Collaboration review concluded that there was no evidence to recommend the use of transcutaneous CO2 monitoring in neonates (53).
In critically ill patients, the PcCO2 gap might be of value as an additional resuscitation endpoint when other parameters (e.g., arterial lactate and ScVO2) are in the normal range despite persistent tissue hypoperfusion (54). However, well-designed, adequately powered, randomized, controlled studies of the efficacy and safety of transcutaneous CO2 monitoring are now required.
Bladder PCO2 (PbCO2)
The results of several animal studies have suggested that monitoring the intramucosal PCO2 in the bladder (PbCO2) may be a minimally invasive technique for monitoring perfusion (55-57). This technique measures PbCO2 via the gas analysis of saline samples collected from the balloon of a Foley catheter inserted into the bladder. The PbCO2 value is well correlated with DO2 and PgCO2 (55,56). However, these results were not confirmed by another group of researchers (57). Clinical studies of the accuracy of this device are required.
Conclusions
Conventional whole-body hemodynamic markers cannot always predict tissue hypoperfusion. By analogy with measurement of the whole-body PCO2 gap, the tissue PCO2 gap has been described as a marker of blood flow adequacy and can be used to detect tissue hypoperfusion. Monitoring PgCO2 gap has given good results, although several technical limitations and failure to develop this tool commercially has prevented the wider use of this technique. Further studies are needed to assess the efficacy and safety of PslCO2 gap and PcCO2 measurements. Although measurement in the bladder are promising, PbCO2 must be now studied in patients. Lastly, the blood flow distribution across the various organs cannot yet be assessed.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Schumacker PT, Cain SM. The concept of a critical oxygen delivery. Intensive Care Med 1987;13:223-9. [Crossref] [PubMed]
- Ackland GL, Iqbal S, Paredes LG, et al. Individualised oxygen delivery targeted haemodynamic therapy in high-risk surgical patients: a multicentre, randomised, double-blind, controlled, mechanistic trial. Lancet Respir Med 2015;3:33-41. [Crossref] [PubMed]
- Bartha E, Arfwedson C, Imnell A. Randomized controlled trial of goal-directed haemodynamic treatment in patients with proximal femoral fracture. Br J Anaesth 2013;110:545-53. [Crossref] [PubMed]
- Grocott MP, Dushianthan A, Hamilton MA, et al. Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev 2012;11:CD004082. [PubMed]
- Fischer MO, Bonnet V, Lorne E, et al. Assessment of macro- and micro-oxygenation parameters during fractional fluid infusion: A pilot study. J Crit Care 2017;40:91-8. [Crossref] [PubMed]
- Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994;330:1717-22. [Crossref] [PubMed]
- Krafft P, Steltzer H, Hiesmayr M, et al. Mixed Venous Oxygen Saturation in Critically III Septic Shock Patients: The Role of Defined Events. Chest 1993;103:900-6. [Crossref] [PubMed]
- Futier E, Robin E, Jabaudon M, et al. Central venous O 2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk surgery. Crit Care 2010;14:R193. [Crossref] [PubMed]
- Mekontso-Dessap A, Castelain V, Anguel N, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med 2002;28:272-7. [Crossref] [PubMed]
- Mallat J, Lemyze M, Meddour M, et al. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care 2016;6:10. [Crossref] [PubMed]
- He HW, Liu DW, Long Y, et al. High central venous-to-arterial CO2 difference/arterial-central venous O2 difference ratio is associated with poor lactate clearance in septic patients after resuscitation. J Crit Care 2016;31:76-81. [Crossref] [PubMed]
- Ospina-Tascón GA, Umaña M, Bermúdez W, et al. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med 2015;41:796-805. [Crossref] [PubMed]
- Nakagawa Y, Weil MH, Tang W, et al. Sublingual Capnometry for Diagnosis and Quantitation of Circulatory Shock. Am J Respir Crit Care Med 1998;157:1838-43. [Crossref] [PubMed]
- Tang W, Weil MH, Sun S, et al. Gastric intramural PCO2 as monitor of perfusion failure during hemorrhagic and anaphylactic shock. J Appl Physiol 1994;76:572-7. [Crossref] [PubMed]
- Marik PE, Bankov A. Sublingual capnometry versus traditional markers of tissue oxygenation in critically ill patients. Crit Care Med 2003;31:818-22. [Crossref] [PubMed]
- Levy B, Gawalkiewicz P, Vallet B, et al. Gastric capnometry with air-automated tonometry predicts outcome in critically ill patients Crit Care Med 2003;31:474-80. [Crossref] [PubMed]
- Randall HM, Cohen J. Anaerobic CO2 production by dog kidney in vitro. Am J Physiol 1966;211:493-505. [Crossref] [PubMed]
- Rackow EC, Astiz ME, Mecher CE, et al. Increased venous-arterial carbon dioxide tension difference during severe sepsis in rats. Crit Care Med 1994;22:121-5. [Crossref] [PubMed]
- Dubin A, Estenssoro E, Murias G, et al. Intramucosal-Arterial Pco2 Gradient Does Not Reflect Intestinal Dysoxia in Anemic Hypoxia J Trauma 2004;57:1211-7. [Crossref] [PubMed]
- Dubin A, Murias G, Estenssoro E, et al. Intramucosal-arterial PCO2 gap fails to reflect intestinal dysoxia in hypoxic hypoxia. Crit Care 2002;6:514-20. [Crossref] [PubMed]
- Weil MH, Rackow EC, Trevino R, et al. Difference in Acid-Base State between Venous and Arterial Blood during Cardiopulmonary Resuscitation. N Engl J Med 1986;315:153-6. [Crossref] [PubMed]
- Zhang H, Vincent JL. Arteriovenous Differences in PCO2 and pH are Good Indicators of Critical Hypoperfusion. Am Rev Respir Dis 1993;148:867-71. [Crossref] [PubMed]
- Groeneveld AB, Vermeij CG, Thijs LG. Arterial and mixed venous blood acid-base balance during hypoperfusion with incremental positive end-expiratory pressure in the pig. Anesth Analg 1991;73:576-82. [PubMed]
- Mecher CE, Rackow EC, Astiz ME, et al. Venous hypercarbia associated with severe sepsis and systemic hypoperfusion. Crit Care Med 1990;18:585-9. [Crossref] [PubMed]
- Bakker J, Vincent JL, Gris P, et al. Veno-arterial Carbon Dioxide Gradient in Human Septic Shock. Chest 1992;101:509-15. [Crossref] [PubMed]
- Vallet B, Teboul JL, Cain S, et al. Venoarterial CO2 difference during regional ischemic or hypoxic hypoxia. J Appl Physiol 2000;89:1317-21. [Crossref] [PubMed]
- Bennett-Guerrero E, Panah MH, Bodian CA, et al. Automated Detection of Gastric Luminal Partial Pressure of Carbon Dioxide during Cardiovascular Surgery Using the Tonocap. Anesthesiology 2000;92:38. [Crossref] [PubMed]
- Nelson DP, Beyer C, Samsel RW, et al. Pathological supply dependence of O2 uptake during bacteremia in dogs. J Appl Physiol 1987;63:1487-92. [Crossref] [PubMed]
- Hamilton-Davies C, Mythen MG, Salmon JB, et al. Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometry. Intensive Care Med 1997;23:276-81. [Crossref] [PubMed]
- Dubin A, Estenssoro E, Murias G, et al. Effects of hemorrhage on gastrointestinal oxygenation. Intensive Care Med 2001;27:1931-6. [Crossref] [PubMed]
- Marik PE. Regional carbon dioxide monitoring to assess the adequacy of tissue perfusion. Curr Opin Crit Care 2005;11:245. [Crossref] [PubMed]
- Lebuffe G, Decoene C, Pol A, et al. Regional Capnometry with Air-Automated Tonometry Detects Circulatory Failure Earlier Than Conventional Hemodynamics After Cardiac Surgery. Anesth Analg 1999;89:1084-90. [Crossref] [PubMed]
- Silva E, De Backer D, Creteur J, et al. Effects of fluid challenge on gastric mucosal PCO 2 in septic patients. Intensive Care Med 2004;30:423-9. [Crossref] [PubMed]
- Creteur J. Gastric and sublingual capnometry. Curr Opin Crit Care 2006;12:272. [Crossref] [PubMed]
- Sato Y, Weil MH, Tang W, et al. Esophageal PCO2 as a monitor of perfusion failure during hemorrhagic shock. J Appl Physiol 1997;82:558-62. [Crossref] [PubMed]
- Totapally BR, Fakioglu H, Torbati D, et al. Esophageal capnometry during hemorrhagic shock and after resuscitation in rats. Crit Care 2003;7:79-84. [Crossref] [PubMed]
- Creteur J, De Backer D, Sakr Y, et al. Sublingual capnometry tracks microcirculatory changes in septic patients. Intensive Care Med 2006;32:516-23. [Crossref] [PubMed]
- Marik PE. Sublingual Capnography: A Clinical Validation Study. Chest 2001;120:923-7. [Crossref] [PubMed]
- Rackow EC, O’Neil P, Astiz ME, et al. Sublingual Capnometry and Indexes of Tissue Perfusion in Patients With Circulatory Failure. Chest 2001;120:1633-8. [Crossref] [PubMed]
- Maciel AT, Creteur J, Vincent JL. Tissue capnometry: does the answer lie under the tongue? Intensive Care Med 2004;30:2157-65. [Crossref] [PubMed]
- Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32:1825-31. [Crossref] [PubMed]
- De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002;166:98-104. [Crossref] [PubMed]
- Jin X, Weil MH, Sun S, et al. Decreases in organ blood flows associated with increases in sublingual PCO2 during hemorrhagic shock. J Appl Physiol 1998;85:2360-4. [Crossref] [PubMed]
- Povoas HP, Weil MH, Tang W, et al. Comparisons Between Sublingual and Gastric Tonometry During Hemorrhagic Shock. Chest 2000;118:1127-32. [Crossref] [PubMed]
- Weil MH, Nakagawa Y, Tang W, et al. Sublingual capnometry: A new noninvasive measurement for diagnosis and quantitation of severity of circulatory shock. Crit Care Med 1999;27:1225-9. [Crossref] [PubMed]
- Eberhard P. The Design, Use, and Results of Transcutaneous Carbon Dioxide Analysis: Current and Future Directions Anesth Analg 2007;105:S48-52. [Crossref] [PubMed]
- Bendjelid K, Schütz N, Stotz M, et al. Transcutaneous Pco2 monitoring in critically ill adults: Clinical evaluation of a new sensor. Crit Care Med 2005;33:2203-6. [Crossref] [PubMed]
- Maniscalco M, Zedda A, Faraone S, et al. Evaluation of a transcutaneous carbon dioxide monitor in severe obesity. Intensive Care Med 2008;34:1340-4. [Crossref] [PubMed]
- Gancel PE, Roupie E, Guittet L, et al. Accuracy of a transcutaneous carbon dioxide pressure monitoring device in emergency room patients with acute respiratory failure. Intensive Care Med 2011;37:348-51. [Crossref] [PubMed]
- Lermuzeaux M, Meric H, Sauneuf B, et al. Superiority of transcutaneous CO2 over end-tidal CO2 measurement for monitoring respiratory failure in nonintubated patients: A pilot study. J Crit Care 2016;31:150-6. [Crossref] [PubMed]
- Lambert LL, Baldwin MB, Gonzalez CV, et al. Accuracy of Transcutaneous CO2 Values Compared With Arterial and Capillary Blood Gases. Respir Care 2018;63:907-12. [Crossref] [PubMed]
- Vallée F, Mateo J, Dubreuil G, et al. Cutaneous ear lobe Pco2 at 37°C to evaluate microperfusion in patients with septic shock. Chest 2010;138:1062-70. [Crossref] [PubMed]
- Bruschettini M, Romantsik O, Zappettini S, et al. Transcutaneous carbon dioxide monitoring for the prevention of neonatal morbidity and mortality. Cochrane Database Syst Rev 2016;2:CD011494. [PubMed]
- Mallat J, Vallet B. Mucosal and cutaneous capnometry for the assessment of tissue hypoperfusion. Minerva Anestesiol 2018;84:68-80. [PubMed]
- Dubin A, Pozo MO, Edul VSK, et al. Urinary bladder partial carbon dioxide tension during hemorrhagic shock and reperfusion: an observational study. Crit Care 2005;9:R556-61. [Crossref] [PubMed]
- Morgaz J, Espigares-Rodríguez L, Muñoz-Rascón P, et al. Evaluation of gastric and bladder tonometry as indicators of tissue perfusion in induced hypotension in dogs: Gastric and vesical tonometry in dogs. J Vet Emerg Crit Care (San Antonio) 2017;27:532-8. [Crossref] [PubMed]
- Seller-Pérez G, Herrera-Gutiérrez ME, Aragón-González C, et al. Bladder Mucosal CO2 Compared with Gastric Mucosal CO2 as a Marker for Low Perfusion States in Septic Shock. ScientificWorldJournal 2012;2012:360378. [Crossref] [PubMed]