
Initial experiences with a centrifugal-pump based minimal invasive extracorporeal circulation system in pediatric congenital cardiac surgery
Introduction
The correction of the majority of congenital cardiac defects requires the implementation of an extracorporeal circulation circuit (ECC). Currently, the applied circuits for ECC in pediatric cardiac surgery are similar to the systems used in adult cardiac surgery, consisting of the basic components: a roller pump, an oxygenator, an open gravity-depending blood reservoir for venous return and shed-blood managing. Although this system provides adequate and safe hemodynamic support for the wide spectrum of corrective surgical procedures in pediatric patients, it is associated with multiple significant adverse effects (1-3). Of particular concern are the detrimental effects to the coagulation system and the provocation of an acute systemic inflammatory reaction (4,5). These negative effects mostly occur in response to the blood-artificial surface and blood-air contact, the mechanical damage to the blood components, the consumption of coagulation factors with subsequent transfusion requirements (5,6). The systemic inflammatory response syndrome presents in a reduced postoperative clinical status with cardiopulmonary and renal dysfunction, increased microvascular permeability leading to edema and tissue malperfusion, which can ultimately result in multiorgan failure, and which is particularly pronounced in infants and neonates (6,7).
In order to minimize the adverse effects and improve end-organ protection minimal invasive extracorporeal circulation (MiECC) systems are successfully applied. Multiple studies have demonstrated the clinical benefits of MiECC systems, such as reduced hemodilution, reduced postoperative bleeding and transfusion requirements (3,8,9), preserved renal function (10,11), improved myocardial protection (12-14), attenuated inflammatory response (6,7), enhanced recovery of microvascular organ perfusion (15-17).
However, all this evidence is deriving from studies performed in adult patients with the majority being coronary artery bypass grafting and aortic valve replacement procedures. Providing those positive effects, the MiECC perfusion concept appears particularly of interest for pediatric cardiac surgery. However, so far, there are no reports on the clinical application of a MiECC system for corrective surgery in neonates and children.
Here we report our initial experiences by using a MiECC system in pediatric cardiac surgery.
Methods
The system specification and requirements were determined to be:
- Allowance to perform closed and open-heart procedures;
- Adequate perfusion of patients from neonate to adult size;
- A modular set-up.
Following the classification of MiECC circuits by the Minimal invasive Extra-Corporeal Technologies International Society (MiECTiS), type I and type III perfusion circuits were assembled depending on the planned intervention: type I was applied for closed heart interventions while type III was used for open heart procedures (9).
The MiECC type I consists of a closed circuit, which includes the oxygenator, the pump and tubing lines. The circuit has no open venous reservoir. All components of the minimal invasive extracorporeal circuits are coated with heparin and the tubing system is significantly reduced in length. In closed heart procedures e.g., central aortic-pulmonary shunt procedures it is possible to reduce the MiECC systems to a minimum (9). For management of intraoperative shed-blood the use of autologous cell saver transfusion system is required.
The main difference between MiECC type l und MiECC type III is the improved capability of shed-blood management. This is achieved by different modular configurations: First, the installation of a reservoir in the MiECC circuit. Second, the integration of additional cardiotomy suction and venting lines (Figure 1).

The MiECC circuits for type I and type III consisted of ¼ - ¼Trilium® coated tubing (Medtronic Inc., Minneapolis, MN USA) with the Affinity® centrifugal blood pump (Medtronic Inc., Minneapolis, MN USA) and a hydrophobic oxygenation membrane CAPIOX® FX 05 for <10 kg bodyweights (Terumo© Cardiovascular Systems Corporation, MI, USA), for 10 to 20 kg Affinity® oxygenator system (Medtronic Inc., Minneapolis, MN USA) or CAPIOX® FX 15 for 20–50 kg (Terumo© Cardiovascular Systems Corporation, MI, USA).
The MiECC priming consisted of Ringer’s Lactate mixture and red-blood-cells in selected cases with a hematocrit <25%.
Surgical technique
Surgical interventions were performed in a standardized fashion as required for the correction of the underlying malformation. Either a median sternotomy (MS) was applied or in selected cases a vertical right axillary thoracotomy (VRAMT) for atrial septal or ventricular septal defects (VSDs).
While surgical shunt implantations, partial cavopulmonary connections (PCPC) and total cavopulmonary connection (TCPC) procedures were performed on a closed, beating heart; right ventricle-to-pulmonary artery (RV-PA) conduit changes were performed with a beating heart, and corrections of atrial septal defects (ASD) type II, VSDs and partial atrioventricular septal defects (PAVSD) were corrected during short episodes of induced fibrillation. For all procedures, the surgical field was routinely flooded with continuous CO2 insufflation. MiECC was established following aortic and venous cannulation in a normal fashion by using DLP® cannulas (Medtronic Inc, Minneapolis, MN USA) In selected cases percutaneous venous femoral cannulation was performed in the setting of a bi-caval venous cannulation (BIO-MEDICUS®, Medtronic Inc., Pediatric-Venous-Cannulae). During the set-up of the MiECC system, attention was given to a bubble-free connection between the cannulas and the tubing. Venous cannulas were retrograde flushed and filled. A supplemental video demonstrates the correction of a tetralogy of fallot by using a MiECC type III circuit (Figure S1).

Anticoagulation was achieved by administration of a heparin bolus of 400 IE/kg aiming an activated clotting time (ACT) of 450–500 seconds (9,18). All procedures were performed at normothermia, beside body temperature was lowered to mild hypothermia when cardioplegic arrest was applied. The utilized cardioplegia consisted of a single-shot crystalloid cardioplegia (Cardioplexol™) with a volume concentration of 1.5 mL/kg bodyweight as reported previously (19).
The perfusion parameters were monitored in a standardized fashion by invasive arterial and central venous pressure (CVP) measurement with targeting a mean arterial pressure of 40–50 mmHg and a CVP below 10 mmHg. Cerebral perfusion was monitored by using cerebral-near-infrared-spectroscopy (NIRS). Blood gas sampling was performed at regular intervals perioperatively. Transfusion targets were set to a lower limit with a hematocrit of 25% and a hemoglobin level of 80 g/L (20).
Transesophageal echocardiography (TEE) was routinely performed for evaluation of cardiac performance and the results of the surgical correction. The postoperative management followed standardized routine treatment protocols.
Statistical analysis
All operated patients by MiECC were included in the analysis. Missing data were not included in the final statistics (accounting for less than 2%). Variables are presented as numbers with % or as median with min-max ranges and of categorical variables are displayed as frequency distributions (n) and simple percentages (%).
Results
MiECC perfusion was successfully performed in 38 patients. Median patient age was 9.5 months (range, 0.2–176 months) with a median weight of 8.1 kg (range, 2.3–49 kg). MiECC type I was applied for closed heart interventions, such as aortopulmonary shunts (13%) and PCPC (5%). MiECC type III was applied for correction of VSD (32%), ASD (8%), pulmonary artery patch plasty (18%), RV-PA conduit implantation (11%), TCPC (5%), PAVSD (3%), subaortic valvular stenosis resection (3%) and aortic valve reconstruction (3%). Table 1 shows a summary of preoperative demographics.
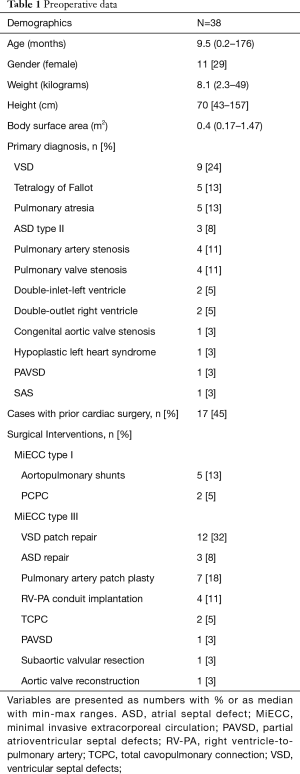
Full table
A MS was applied in the majority of cases (72%) and a vertical right axillary thoracotomy in 11 (28%) cases. Peripheral percutaneous venous cannulation for the inferior caval vein was performed in 9 cases (24%).
For both MiECC types no system related technical complications were encountered. Related to the patients’ body surface area (BSA) adequate flows were at a median of 1.2 L/min (range, 0.5–3.6 L/min) with a target mean arterial pressure between 40 to 50 mmHg at normothermia. The MiECC perfusion times were at a median of 53.5 min (range, 19–117 min). Beating heart procedures were performed in 23 cases (60%) at normothermia, while at 15 interventions cardioplegic cardiac arrest was induced at mild hypothermia. The cross-clamp times varied from 15 to 55 min with a median of 29 min (Table 2).
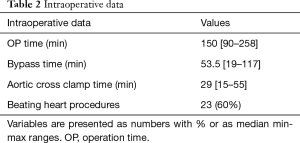
Full table
The post MiECC lactate levels were recorded between with a median of 1.9 (range, 0.7–4.8). Levels of hematocrit were at a median of 34% (range, 28–53%) before MiECC and at a median of 27% (range, 21–43%) after weaning from MiECC. Hemoglobin levels were at a median of 109 g/L (range, 91–164 g/L) before MiECC and at a median of 105 g/L (range, 77–151 g/L) after weaning from MiECC.
All patients had an uneventful perioperative course with no in-hospital mortality. Major adverse cardiac and cerebrovascular events (MACCE) did not occur in the hospitalization period.
Discussion
As previously mentioned, the application of the MiECC perfusion concept to pediatric cardiac surgery might allow for improved perioperative outcome, similar to the positive effects and results demonstrated in adult cardiac surgery. Here we report the first successful application of a MiECC system customized for closed and open pediatric cardiac surgery. The system was working reliable and no conversion to CECC was necessary. All corrective surgical procedures could be performed in a standardized fashion for a variety of malformations with good results and uneventful postoperative course. The modular concept of the system allowed for the adjustment of the perfusion system to the requirement of the intervention. Thus, procedures on a beating closed heart, such as the implantation of a central aorto-pulmonary shunt can be performed by applying a MiECC type I. The application of such a completely closed circuit presents an appealing and attractive solution with the potential for significant benefits for the patients. Thus, particularly due to the fact, that the majority of the patients undergoing shunt procedures present newborns with a bodyweight between 2.5–3.5 kg.
For all open-heart procedures, a reservoir had to be added for venting and suction blood management. Certainly, the resulting MiECC type III signifies a drawback towards the concept of creating a closed perfusion system with as minimalized blood-air contact as possible. Our group is currently investigating into the development of a venting and blood suction system without a hard-shell reservoir. This system should allow for minimizing air aspiration and subsequent air elimination prior to direct re-transfusion into the closed perfusion circuit (9).
While a continuous adequate perfusion was achieved by applying standard cannulation techniques and materials, we would like to address a typical MiECC associated phenomenon: the adjustment of the venous suction drainage, particularly at the start of the perfusion, requires special attention by the cardio-technician and surgeon. Prior to embarking on cross-clamping and cardioplegia administration, time has to be provided in order to establish a balanced and stable perfusion on the MiECC circuit. Especially in the setting of bi-caval cannulation in infants, we observed the occurrence of an imbalanced drainage mostly due to the intermittent collapse of the superior caval vein. Management of this phenomena consisted of the partial clamping of the non-corresponding inferior venous cannula (IVC) or a short reduction of the pump speed. The application of these maneuvers led to a stable perfusion with a balanced venous drainage and the avoidance of additional volume bolus.
Regarding volume management, an advantageous component of our MiECC concept presents the type of applied cardioplegia. The low-volume single shot crystalloid cardioplegia is in line with the principles of MiECC by ensuring limited hemodilution and transfusion requirements. As previously reported, this cardioplegia provides an adequate cardioprotection for cross clamp times of up to 60 min (12,21).
It has to be acknowledged, that proof of a beneficial effect by using a MiECC circuit for pediatric cardiac surgery cannot be answered by this report and needs further investigation. So far, the primary goal was to evaluate the technical feasibility of a MiECC system in neonates up to teenaged patients. Given our experiences, we conclude that MiECC perfusion systems are feasible for closed and open congenital cardiac interventions by using standard techniques and good surgical results.
Until now, almost all advocated positive results and beneficial effects of using a MiECC system derive from adult cardiac surgery. The only studies evaluating the effects of a MiECC system towards its performance in pediatric patients present two animal studies in rabbits. Schnoering et al. compared the inflammatory reaction following the application of a newly developed MiECC type III system against a roller-pump-based ECC. The authors observed less activation of immunological markers, such as IL-6, IL-8, IL-10, and an improved balanced between IL-10/IL-6 ratio, for rabbits of 4.04±0.52 kg following perfusion with a MiECC system (22). In a study by Schnoering et al. less consumption of fibrinogen and lower levels of plasma-free hemoglobin were observed following MiECC perfusion (23). Beside those findings derive from rather small study samples, they are in accordance with the previously mentioned significant positive effects demonstrated for adult cardiac patients undergoing MiECC perfusion (24).
Although appearing to be a detail, we also believe, that a significant technical advantage by using a MiECC system presents the positioning option of the ECC components in the very near proximity to the patient at the operating table. This can allow for a significant further reduction of the tubing length and priming volume of the perfusion system, which might be not possible by using an open gravity-depending conventional ECC system.
Study limitations
We acknowledge some limitations of our study. It was a retrospective observational analysis and therefore cause and effect are hard to establish.
Conclusions
MiECC can be performed by using standard techniques for closed and open cardiac procedures for the correction of a variety of malformations in neonates and children with good results and uneventful postoperative course.
Acknowledgments
The authors would like to thank the cardiotechnicians Martin Schrag, Etienne Zermatten and Anne-Kathrin Beese for their help and assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: According to hospital policy the patients or their relatives when younger than 18 years signed at admission a general informed consent for data collection and regular follow-up for the purpose of medical research.
References
- Anastasiadis K, Asteriou C, Deliopoulos A, et al. Haematological effects of minimized compared to conventional extracorporeal circulation after coronary revascularization procedures. Perfusion 2010;25:197-203. [Crossref] [PubMed]
- Reineke D, Winkler B, König T, et al. Minimized extracorporeal circulation does not impair cognitive brain function after coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2015;20:68-73. [Crossref] [PubMed]
- Kowalewski M, Pawliszak W, Raffa GM, et al. Safety and efficacy of miniaturized extracorporeal circulation when compared with off-pump and conventional coronary artery bypass grafting: evidence synthesis from a comprehensive Bayesian-framework network meta-analysis of 134 randomized controlled trials involving 22,778 patients. Eur J Cardiothorac Surg 2016;49:1428-40. [Crossref] [PubMed]
- Puehler T, Haneya A, Philipp A, et al. Minimized extracorporeal circulation system in coronary artery bypass surgery: a 10-year single-center experience with 2243 patients. Eur J Cardiothorac Surg 2011;39:459-64. [Crossref] [PubMed]
- Kofidis T, Baraki H, Singh H, et al. The minimized extracorporeal circulation system causes less inflammation and organ damage. Perfusion 2008;23:147-51. [Crossref] [PubMed]
- Vohra HA, Whistance R, Modi A, et al. The inflammatory response to miniaturised extracorporeal circulation: a review of the literature. Mediators Inflamm. 2009;2009:707042. [Crossref] [PubMed]
- Farag M, Patil NP, Sabashnikov A, et al. Comparison of Two Miniaturized Cardiopulmonary Bypass Systems Regarding Inflammatory Response. Artif Organs 2017;41:139-45. [Crossref] [PubMed]
- Winkler B, Heinisch PP, Gahl B, et al. Minimally Invasive Extracorporeal Circulation Circuit Is Not Inferior to Off-Pump Coronary Artery Bypass Grafting: Meta-Analysis Using the Bayesian Method. Ann Thorac Surg 2017;103:342-50. [Crossref] [PubMed]
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016;22:647-62. [Crossref] [PubMed]
- Deininger S, Hoenicka M, Müller-Eising K, et al. Renal Function and Urinary Biomarkers in Cardiac Bypass Surgery: A Prospective Randomized Trial Comparing Three Surgical Techniques. Thorac Cardiovasc Surg 2016;64:561-8. [PubMed]
- Anastasiadis K, Antonitsis P, Deliopoulos A, et al. A multidisciplinary perioperative strategy for attaining “more physiologic” cardiac surgery. Perfusion 2017;32:446-53. [Crossref] [PubMed]
- Immer FF, Pirovino C, Gygax E, et al. Minimal versus conventional cardiopulmonary bypass: assessment of intraoperative myocardial damage in coronary bypass surgery. Eur J Cardiothorac Surg 2005;28:701-4. [Crossref] [PubMed]
- Haneya A, Philipp A, Diez C, et al. Myocardial protection in patients undergoing coronary artery bypass grafting surgery using minimized extracorporeal circulation in combination with volatile anesthetic. Asaio J 2010;56:532-7. [Crossref] [PubMed]
- Zangrillo A, Garozzo FA, Biondi-Zoccai G, et al. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: A meta-analysis of randomized controlled studies. J Thorac Cardiovasc Surg 2010;139:1162-9. [Crossref] [PubMed]
- Rufa M, Schubel J, Ulrich C, et al. A retrospective comparative study of minimally invasive extracorporeal circulation versus conventional extracorporeal circulation in emergency coronary artery bypass surgery patients: a single surgeon analysis. Interact Cardiovasc Thorac Surg 2015;21:102-7. [Crossref] [PubMed]
- El-Essawi A, Breitenbach I, Haupt B, et al. Impact of minimally invasive extracorporeal circuits on octogenarians undergoing coronary artery bypass grafting. Have we been looking in the wrong direction? Eur J Cardiothorac Surg 2017;52:1175-81. [Crossref] [PubMed]
- Anastasiadis K, Asteriou C, Antonitsis P, et al. Enhanced recovery after elective coronary revascularization surgery with minimal versus conventional extracorporeal circulation: a prospective randomized study. J Cardiothorac Vasc Anesth 2013;27:859-64. [Crossref] [PubMed]
- Bauer A, Hausmann H, Schaarschmidt J, et al. Is 300 Seconds ACT Safe and Efficient during MiECC Procedures? Thorac Cardiovasc Surg 2019;67:191-202. [Crossref] [PubMed]
- Kairet K, Deen J, Vernieuwe L, et al. Cardioplexol, a new cardioplegic solution for elective CABG. J Cardiothorac Surg 2013;8:120. [Crossref]
- Cholette JM, Faraoni D, Goobie SM, et al. Patient Blood Management in Pediatric Cardiac Surgery. Anesth Analg 2018;127:1002-16. [Crossref] [PubMed]
- Immer FF, Ackermann A, Gygax E, et al. Minimal extracorporeal circulation is a promising technique for coronary artery bypass grafting. Ann Thorac Surg 2007;84:1515-20; discussion1521. [Crossref] [PubMed]
- Schnoering H, Arens J, Terrada E, et al. A newly developed miniaturized heart-lung machine--expression of inflammation in a small animal model. Artif Organs 2010;34:911-7. [Crossref] [PubMed]
- Schnoering H, Arens J, Sachweh JS, et al. The Aachen miniaturized heart-lung machine--first results in a small animal model. Artif Organs 2009;33:935-40. [Crossref] [PubMed]
- Rahe-Meyer N, Solomon C, Tokuno ML, et al. Comparative assessment of coagulation changes induced by two different types of heart-lung machine. Artif Organs 2010;34:3-12. [Crossref] [PubMed]
- Kadner A, Heinisch PP, Bartkevics M, et al. Intraoperative video demonstration of a MiECC type III circuit for the correction for Tetralogy of Fallot. Asvide 2019;6:174. Available online: http://www.asvide.com/article/view/32287


