
Multidisciplinary team approach on massive postpartum pulmonary thromboembolism: experience from three cases
Introduction
Thromboembolic events are one of the leading causes of maternal death during the postpartum period. In developed countries, the maternal death rate due to thromboembolic events is 1.34 per 100,000 live births, accounting for 14.9% of all maternal deaths (1). The incidence of pulmonary thromboembolism (PTE), a serious clinical condition that results in death of the mother either during pregnancy or the peripartum period, was reported as 1 in 1,000–3,000 pregnancies, and PTE is the leading cause of maternal death, with a rate of about 5%, in industrialized countries (2-4). A Maternal death exploratory committee in Japan reported that 13 out of 213 maternal deaths between 2010 and 2012 were due to pulmonary embolism (5). In a Korean single-center report, there were 13 cases of pulmonary embolism out of a total of 57,092 deliveries over an eight-year period. The authors stated that all cases occurred during the postpartum period after delivery with Cesarean section (C/S) and that ten patients were diagnosed during the early postpartum period within the first 48 hours of delivery. Of these 13 patients, ten improved with only anticoagulation therapy, whereas two patients received surgical thrombectomy, and one patient died (6).
A major hypothesis delineating the pathogenesis of venous thromboembolism (VTE), often called Virchow’s triad, proposes that VTE occurs because of alteration in blood flow (stasis), vascular endothelial injury and hypercoagulable state. Pregnancy is associated with an increased risk of thrombosis that might be due in part to obstruction of venous return by the enlarged uterus as well as the hypercoagulable state associated with pregnancy. C/S is associated with vascular injury and immobilization, thereby increasing the risk of VTE.
Previous case reports and reviews reported successful results in these circumstances (7-10). For example, a systematic review by Martillotti et al. (7) reported that the maternal survival rate was as high as 86.1–100% in patients with massive PTE during pregnancy and the postpartum period who were treated by various modalities including thrombolysis, surgical embolectomy, percutaneous thrombectomy and extracorporeal membrane oxygenation (ECMO). A review by Akazawa et al. (8) including 13 patients who received thrombolysis with intravenous recombinant tissue plasminogen activator during the early postpartum period revealed that ten patients survived. Despite the reportedly high survival rates, the incidence of major bleeding is notably high in postpartum patients, especially those undergoing C/S. Martillotti et al. (7) reported that the incidence rates of major bleeding after thrombolysis were 58.3% and 17.5% in the postpartum period and during pregnancy, respectively. Akazawa’s review included eight patients who delivered by C/S, all of whom were transfused after thrombolysis; four out of the seven patients with available medical records, hysterectomy or hematoma-removal surgery was performed due to bleeding.
In clinical practice, saving patients from this life-threatening situation is very difficult. The reported case series included patients with successful outcomes; therefore, survival rates were higher than expected, creating a publication bias because only successful cases are reported, and mortality cases tend to be ignored.
An immediate decision for treatment and a multidisciplinary team approach are necessary for these patients. We recently experienced three catastrophic cases of patients with PTE after C/S, two of whom died despite receiving aggressive management. We herein report some of the important lessons about the practical challenges we experienced during the management of these three patients and discuss the pitfalls and approaches for better treatment plans with international multidisciplinary experts.
Case presentation
Case 1 (November 2014)
A 43-year-old female underwent her second C/S at a local obstetric clinic one day before presenting to our emergency room (ER). At 9:30 pm, she felt dyspnea and epigastric pain and was determined to be hypotensive. The obstetrician suspected pulmonary edema, and the patient was transferred to our hospital. At 11:00 pm, she arrived at the ER. The patient’s initial vital signs were as follows: blood pressure, 127/93 mmHg; heart rate, 153 beats per minute; respiratory rate, 34 breaths/minute; body temperature, 36.5 °C; and SpO2, 90%. Oxygen (5 L/minute) was administered through an oxygen mask. She had no medical or familial history. There was no medical record of prophylaxis for deep vein thrombosis (DVT) administered by the local clinic. Chest X-ray showed no active lung lesions. After 10 minutes, her blood pressure dropped to 68/53 mmHg, and she went into cardiac arrest 30 minutes later. A chest computed tomography (CT) scan could not be performed because of her condition, and cardiac echography showed right ventricular enlargement and a D-shaped left ventricle.
Case 2 (November 2016)
A 33-year-old female was admitted to our ER after C/S performed at a local clinic earlier in the day at 1:00 p.m. The patient experienced dizziness and dyspnea 9 hours later and had a seizure attack; cardiac arrest occurred 30 minutes later at the local clinic.
Cardiopulmonary cerebral resuscitation (CPCR) was performed repeatedly. Her medical history was unremarkable, and we could not obtain medical information on DVT prophylaxis by the local clinic. Cardiac echocardiography showed a D-shaped left ventricle.
Case 3 (October 2017)
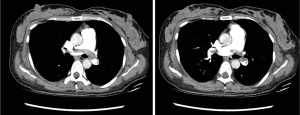
A 39-year-old female received her third C/S four hours before visiting our ER. She developed dyspnea three hours after surgery, and her initial vital signs were as follows: blood pressure, 81/56 mmHg; heart rate, 102 beats per minute; respiratory rate, 24 breaths/minute; body temperature, 36.1 °C; and SpO2, 93%. Oxygen (3 L/minute) was administered through a nasal cannula. Her medical history was unremarkable, and there was no PTE in her previous two C/S surgeries. We could not obtain medical information on DVT prophylaxis by the local clinic. Her blood pressure recovered after 10 minutes, but portable cardiac echography showed a D-shaped left ventricle. Chest CT scan (Figure 1) showed massive PTE in both main pulmonary arteries but no evidence of DVT.
Treatment and follow-up
Case 1 (November 2014)
Thrombolysis and ECMO
The patient was diagnosed with massive PTE and administered 100 mg alteplase by continuous intravenous (IV) infusion. CPCR was performed immediately, and she was supported by venoarterial extracorporeal membrane oxygenation (ECMO). The activated prothrombin time (aPTT) was 38.3 (normal range, 24.0–33.0) seconds initially but could not be determined thereafter due to prolongation. Therefore, heparin was not used. Of note, the ECMO circuit was not heparin-coated. During the procedure, the patient had a seizure and was transferred to the intensive care unit (ICU) where she was administered vasopressors and transfusions; however, the patient showed signs of shock and was bleeding from all IV catheter sites. She had epistaxis, a huge hematoma in the catheter site, intraabdominal bleeding, and intractable vaginal bleeding. She died within 12 hours of admission to our ER.
Case 2 (November 2016)
Percutaneous thrombectomy and embolization
The venoarterial ECMO was initiated, and pulmonary angiography was conducted. The thrombus in the right main pulmonary artery (Figure 2) was removed by catheter thrombectomy. The patient’s aPTT could not be measured due to prolongation; therefore, heparin or enoxaparin was not used. Of note, the ECMO circuit was heparin-coated. Vaginal bleeding was observed during the procedure; therefore, she received transfusions, and both internal iliac arteries were embolized with Gelfoam. The patient was transferred to the ICU where she underwent continuous renal replacement therapy because of anuria. She suffered hematochezia, abdominal distension, and shock and died 36 hours after admission to our ER.

Case 3 (October 2017)
Low-molecular-weight-heparin and surgical hematoma removal
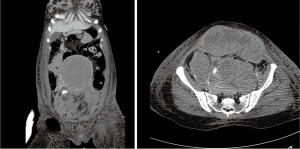
Considering the bleeding risk and the recovered blood pressure, we opted for anticoagulation treatment using only low-molecular-weight-heparin. She was transferred to the ICU and closely monitored. Her vital signs were stable and oxygen (3 L/minute) was administered through a nasal cannula; however, her hemoglobin levels decreased by approximately 2 g/dL daily, and the circumference of her abdomen increased. A total of 13 pints of packed red blood cells were transfused over seven days. The follow-up chest and pelvis CT revealed marked improvement of the thrombus burden in the pulmonary artery but huge hematomas in the uterus, pelvis, and abdominal muscle layer (Figure 3). Low-molecular-weight-heparin was discontinued, a retrievable inferior vena cava filter was implanted, and both uterine arteries and the left inferior epigastric artery were embolized. Finally, hematoma-removal surgery was performed, and the patient was discharged after restarting anticoagulation treatment.
Discussion
Clark et al. (11) conducted a study to determine maternal mortality in the United States and compared the mortality rates from post-C/S pulmonary embolism between 2007–2012 and 2000–2006 periods. They reported that the universal use of pneumatic compression devices in all women who underwent C/S delivery resulted in a decrease in deaths due to postoperative pulmonary embolism from 7 in 458,097 to 1 in 465,880 C/S births (P=0.038). However, information regarding the use of pneumatic compression devices at the local clinic before their transfer to our hospital could not be obtained for any of the three cases. This illustrates the critical significance of promoting the routine use of pneumatic compression devices in patients undergoing C/S at local obstetric clinics in preventing these catastrophic results.
The three cases provide important lessons and highlight the many difficulties faced during the management of postpartum PTE, especially during the period after C/S delivery. First, the postpartum period is fraught with unfavorable circumstances, especially in post-C/S patients, which prompt physicians to question their treatment decision. In post-C/S patients, thrombolysis is contraindicated as a traditional protocol because of the immediate postoperative status; however, several reports support the utility of thrombolytic therapy. The review by Akazawa (8) revealed that transfusion was used in all thirteen patients and that hysterectomy or hematoma-removal surgery was performed after thrombolysis in four of the seven patients of whose medical records could be located. The first patient in the current report suffered from bleeding from all IV catheter sites and intractable vaginal bleeding. One patient suffered from a huge hematoma and required surgery, although only low molecular weight heparin was administered.
The second consideration for treatment of these patients is the optimal dose of thrombolytic agent. The alteplase doses varied from 30 to 175 mg among the patients reported by Akazawa et al. (8). Recent data suggest that lower alteplase doses were equally effective, with less bleeding, in patients with pulmonary embolism (12,13). We suggest a relatively lower dose of alteplase during the post-C/S period.
The third issue in these patients is bleeding. After thrombolysis, cannulation for ECMO, bleeding from catheter insertion sites, and C/S-related surgery-site bleeding are important issues. Two of our patients who died displayed intractable vaginal bleeding. Bleeding might occur even after prophylactic uterine artery embolization because of the dynamic vessel diameter, requiring repetitive embolization. Generally, the obstetrician is wary of hysterectomy or hematoma-removal surgery in patients with massive PTE because of the possibility of death during surgery and might be hesitant to perform such surgeries; however, they should be persuaded to consider using this treatment protocol.
The classic criteria for treatment of PTE, surgical thrombectomy, or percutaneous catheter thrombectomy are suitable for postpartum patients (14,15). The success depends entirely on the experience and skill of the surgeon or the interventionist as the procedures are not performed often and might result in worse outcomes if those involved are not expert.
Finally, the team approach is critical for a favorable outcome. The choice of treatment, immediate intervention, and estimation of the blood loss and required transfusion amount should be simultaneous and require different specialists; therefore, we propose that an emergency team of experts including pulmonologists, cardiologists, chest surgeons, radiologists, and obstetricians must convene to treat postpartum PTE.
iMDT discussion
Surgical embolectomy may be the best option in cases such as those presented here. However, not all hospitals have chest surgeons who can perform embolectomy on staff or on call who can arrive immediately in this urgent situation. What is the next best plan?
Expert opinion 1: Kwok Ming Ho (Department of Intensive Care Medicine)
A management protocol for massive, near fatal, pulmonary embolism is needed for all centers where patients may present with massive pulmonary embolism with hemodynamic instability requiring inotropic and vasopressor support. There is no time to waste to discuss the pros and cons of different options under most of these circumstances. Surgical thromboembolectomy is the most effective and safest method but to be actualized, the center must be well prepared in the set-up, 24 hours per day and 7 days per week, before this is possible for the patients who are already in cardiac arrest. For a center that does not have in-house cardiothoracic surgery to perform surgical thromboembolectomy, there are three not mutually exclusive options—systemic thrombolysis, ECMO and catheter suctioning or fragmentation. Both latter two options again require availability of local expertise and can be used as an alternative to thrombolysis (if the patients are not in cardiac arrest or they have contraindications to thrombolysis). Once cardiac arrest already occurred, there are really only two options, systemic thrombolysis, ECMO or both. ECMO can only be used if there is a primed circuit available 24 hours per day that is ready to go. Without pre-prepared primed ECMO circuit, systemic thrombolytic is the only option followed by ECMO if deemed feasible and necessary.
Expert opinion 2: Laura A. Dalla Vecchia, Cardiologist (Department of Cardiology)
The clinical presentation of the above three cases in the ER was dramatic. PTE is per se a life-threatening condition that is even more complex with the impending postpartum risk of bleeding. Ideally, an emergency team of experts, including chest surgeon, pulmonologist, cardiologist, radiologist, obstetrician, as well as anesthesiologist should convene to treat postpartum PTE. However, the 24-hour availability of such a team is extremely difficult to realize. Whatever clinical staff, a prompt treatment is lifesaver. A standardized up-dated protocol should be followed. Taking a step back, risk stratification and prevention are also extremely important, as well as effective and inexpensive. Indeed, long before childbirth any risk factor, such as thrombophilia, excessive weight, age, personal history of VTE, diabetes, hospital admission, or surgery should be identified and proper medical prophylaxis instituted (2,16). In the postpartum, especially after C/S delivery, early disabling and ambulation, graduated venous compression stockings, or sequential compression devices should be utilized (16). Thus, early diagnosis of DVT/PTE is mandatory. Clinical suspicion must arise in case of any suggestive symptom or sign. A delay in the diagnosis can be fatal. Therefore, if diagnostic tests are not available, anticoagulation treatment using low-molecular-weight-heparin should be started, unless a very high risk of bleeding, and a transfer to a pre-alerted ER should be performed rapidly. A CPCR trained professional should always escort the patient. The details of her personal history, used prophylaxis for DVT, concomitant medications should also be anticipated to the ER in order to accelerate the decision making process. Lastly, each case of postpartum complication is a teaching opportunity. A careful root cause analysis should be performed aimed at identifying possible errors and virtuous actions to improve the diagnostic-therapeutic path (17) in each local setting.
We believe that acute thrombus in patients similar to those presented here can be resolved easily; therefore, maintenance of the vital signs are more important than the efforts for thrombus resolution. We ask the experts if they agree that ECMO plays a critical role in this situation
Expert opinion 1
Yes, ECMO can successfully support patients with massive pulmonary embolism in shock or cardiac arrest without dissolving or breaking the thrombus immediately. The thrombus will resolve with heparinization for the ECMO with time and ECMO can be weaned once the right ventricle has recovered. The difficulty will be whether the center has the expertise and pre-prepared ECMO circuit ready to be initiated during cardiac arrest 24 hours per day, 7 days per week.
Expert opinion 2
No doubt, surgical embolectomy would be the treatment of choice in case of massive PTE when a chest surgical team with specific expertise is available. However, maintenance of the vital signs is crucial for either a surgical or percutaneous thrombectomy, or for medical treatment. I think that ECMO certainly plays a crucial role in this perspective. It would also allow to monitor the evolution of the arterial thrombosis to make the further step.
Akazawa (8) reported that the dose of alteplase varied from 30 to 175 mg among their patients. Considering the high risk of bleeding, what is the optimal alteplase dose for patients in this condition?
Expert opinion 1
This is a risk to benefit judgment question. In patients with pulmonary embolism in cardiac arrest, time to restore spontaneous circulation is critical and hence 100 mg (2 vials) will be a reasonable dose to hopefully restore circulation in the shortest time possible. For those who are only with hypotension requiring moderate to high dose inotropes/vasopressors, an initial dose of 10 mg followed by an infusion of the remaining 90 mg over a couple of hours would appear reasonable.
Expert opinion 2
With regard to the dose of thrombolytic therapy, either alteplase or other recombinant-based plasminogen activators, a reduced dose can be considered in order to balance between safety, i.e., avoiding bleeding, and effectiveness, i.e., dissolving the thrombus. Given the uniqueness of each case presentation, a strict general rule is not advisable. Careful monitoring of the laboratory data, in particular the aPTT, is obviously helpful in guiding the infusion dosage adjustment.
In all three patients presented here, bleeding was a serious issue even though only low molecular weight heparin was administered. What is your opinion about prophylactic embolization of the uterine artery?
Expert opinion 1
I am uncertain whether this will be a better option than with the interventional radiologist on standby ready to initiate embolization immediately if bleeding does occur. An obstetrician may also be helpful if they can insert a balloon vaginally together with manual compression externally (similar to severe atonic uterus bleeding) to tamponade the uterus while the interventional radiologist is embolizing the uterine arteries.
Expert opinion 2
In all three women, bleeding was, not surprisingly, an extremely critical problem. Again, to prevent rather than to treat, in this case the hemorrhage, which is even refractory during anticoagulation, is a main objective. Prophylactic embolization of the uterine artery has been proposed as an alternative to surgical hysterectomy in several randomized controlled trials and observational studies (18). The former appears to be as effective as the latter in reducing postpartum blood loss, and more effective in shortening the operating time. Thus, both options seem viable, the choice should be based on the patient’s anatomical and clinical characteristics, as well as the surgeon’s experience.
In conclusion, facing the dramatic picture of massive pulmonary embolism in the postpartum is extremely complex. A rapid choice is essential, but a wise choice is determinant. This is why a team of experts is desirable, they could act quickly, balancing the risk/benefit ratio, considering all the different medical and surgical options.
Conclusions
Although previous studies reported successful outcomes with very high survival rates, postpartum massive PTE, especially post C/S delivery, is a life-threatening condition with many dilemmas and controversies in management. Two patients were lost, and one patient required surgery due to bleeding after the administration of low-molecular-weight heparin alone. We believe that the treatment for postpartum massive PTE requires an initial team approach including pulmonologists, cardiologists, chest surgeons, radiologists, and obstetricians to choose a tailored treatment option, an aggressive surgery approach, and a protocol for monitoring postpartum bleeding.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the Chungbuk National University Hospital, Republic of Korea.
References
- Cristina Rossi A, Mullin P. The etiology of maternal mortality in developed countries: a systematic review of literature. Arch Gynecol Obstet 2012;285:1499-503. [Crossref] [PubMed]
- Sultan AA, Tata LJ, West J, et al. Risk factors for first venous thromboembolism around pregnancy: a population-based cohort study from the United Kingdom. Blood 2013;121:3953-61. [Crossref] [PubMed]
- Berg CJ, Callaghan WM, Syverson C, et al. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2010;116:1302-9. [Crossref] [PubMed]
- Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. Bjog 2011;118 Suppl 1:1-203. [Crossref] [PubMed]
- Hasegawa J, Sekizawa A, Tanaka H, et al. Current status of pregnancy-related maternal mortality in Japan: a report from the Maternal Death Exploratory Committee in Japan. BMJ Open 2016;6:e010304. [Crossref] [PubMed]
- Lee MY, Kim MY, Han JY, et al. Pregnancy-associated pulmonary embolism during the peripartum period: An 8-year experience at a single center. Obstet Gynecol Sci 2014;57:260-5. [Crossref] [PubMed]
- Martillotti G, Boehlen F, Robert-Ebadi H, et al. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost 2017;15:1942-50. [Crossref] [PubMed]
- Akazawa M, Nishida M. Thrombolysis with intravenous recombinant tissue plasminogen activator during early postpartum period: a review of the literature. Acta Obstet Gynecol Scand 2017;96:529-35. [Crossref] [PubMed]
- Bilger A, Pottecher J, Greget M, et al. Extensive pulmonary embolism after severe postpartum haemorrhage: management with an inferior vena cava filter. Int J Obstet Anesth 2014;23:390-3. [Crossref] [PubMed]
- Goel S, Nath R, Pandit N. Pharmaco-mechanical management of acute massive pulmonary embolism in a postpartum female. Indian Heart J 2014;66:378-81. [Crossref] [PubMed]
- Clark SL, Christmas JT, Frye DR, et al. Maternal mortality in the United States: predictability and the impact of protocols on fatal postcesarean pulmonary embolism and hypertension-related intracranial hemorrhage. Am J Obstet Gynecol 2014;211:32.e1-9. [Crossref] [PubMed]
- Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the "MOPETT" Trial). Am J Cardiol 2013;111:273-7. [Crossref] [PubMed]
- Zhang Z, Zhai ZG, Liang LR, et al. Lower dosage of recombinant tissue-type plasminogen activator (rt-PA) in the treatment of acute pulmonary embolism: a systematic review and meta-analysis. Thromb Res 2014;133:357-63. [Crossref] [PubMed]
- Minakawa M, Fukuda I, Miyata H, et al. Outcomes of Pulmonary Embolectomy for Acute Pulmonary Embolism. Circ J 2018;82:2184-90. [Crossref] [PubMed]
- Fukuda W, Chiyoya M, Taniguchi S, et al. Management of deep vein thrombosis and pulmonary embolism (venous thromboembolism) during pregnancy. Gen Thorac Cardiovasc Surg 2016;64:309-14. [Crossref] [PubMed]
- Kolettis D, Craigo S. Thromboprophylaxis in Pregnancy. Obstet Gynecol Clin North Am 2018;45:389-402. [Crossref] [PubMed]
- Peerally MF, Carr S, Waring J, et al. BMJ Qual Saf 2017;26:417-22. [PubMed]
- Liu Z, Wang Y, Yan J, et al. Uterine artery embolization versus hysterectomy in the treatment of refractory postpartum hemorrhage: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2018.1-13. [PubMed]


