A comparative study of three-dimensional high-definition and two-dimensional high-definition video systems in totally endoscopic mitral valve replacement
Introduction
It has been widely accepted that the development of endoscopy was a milestone in the history of cardiovascular surgery. Over the past two decades, Endoscopic surgery has been increasingly adopted by cardiovascular surgeons in the management of valvular, congenital, and coronary heart disease. Initially, endoscopic cardiac surgery was based on two-dimensional (2D) imaging. However, the development of three-dimensional high-definition (3D-HD) video system with improved depth perception may prove to be superior to the 2D system. At our institution, we began performing 2D endoscopic cardiac surgery in 2010. In June 2013, the 3D-HD image system was installed. To date, over 350 surgeries have been performed under 3D image guidance.
In the literature, 3D image system has proved its superiority to the 2D system in gastrointestinal resections, hysterectomies, and urologic procedures (1). However, to the best of our knowledge, there have been no comparative reports for cardiovascular procedures. This study aims to compare the performance and benefits of the 3D-HD image system with those of 2D-HD imaging in patients undergoing totally endoscopic mitral valve replacement (TEMVR).
Methods
Study design and patient selection
This study was a single-center comparative trial. Clinical data was recorded in a prospective database for minimally invasive cardiac surgery.
Inclusion criteria was symptomatic congestive heart failure due to rheumatic or non-rheumatic mitral valve disease. All patients underwent preoperative examinations, including: chest X-ray, pulmonary function test, arterial blood-gas analysis, electrocardiogram, echocardiography and coronary angiography (for patients aged over 50). All the patients were operated on by the same surgeon using either 2D or 3D image guidance. Exclusion criteria were as follows: previous cardiac surgery; moderate or severe aortic valve insufficiency; compromised lung function with intolerance to single lung ventilation; peripheral vascular lesions not suitable for cannulation or procedures other than single MVR, including concomitant tricuspid valvuloplasty or concomitant ablation for atrial fibrillation. This study was approved by our Institutional Ethics Review Board (no ID number). Informed consent forms were obtained from all patients enrolled.
The primary outcome measures were aortic cross-clamp time and cardiopulmonary bypass time. Secondary outcome measures included postoperative mechanical ventilation time, length of surgical intensive care unit (SICU) stay, length of hospital stay, major complications, and mortality.
3D-HD and 2D-HD image systems
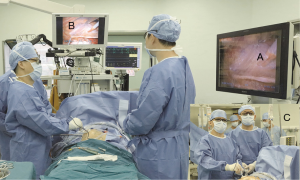
Surgical procedures in the 3D group were performed using the TIPCAM1 Karl Storz 3D system with a 30-degree binary camera head. Surgeons wore polarized glasses to view 3D images on the monitor during the procedure (Figure 1). Surgeries in the 2D group were performed using the Karl Storz Tuttlingen system. Image resolution in each system was identical.
Surgical techniques
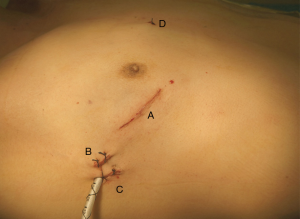
All patients were placed in supine position with the right side slightly elevated. General anesthesia and double-lumen endobronchial intubation were performed. Four skin incisions were made (Figure 2). After systemic heparinization, peripheral cardiopulmonary bypass was established with cannulation of the right femoral artery and vein. The right jugular vein was also cannulated by the anesthesiologist to enhance drainage of the superior vena cava. The patient was cooled to 32 °C before the ascending aorta was cross-clamped. Cold blood cardioplegia was delivered through the aortic root. After cardiac arrest, a direct left atriotomy was performed. Exposure of the mitral valve was facilitated with a self-retaining retractor system. The mitral valve was then resected and replaced with an artificial prosthesis. The internal orifice of the left atrium auricle was closed with running sutures when preoperative atrial fibrillation was present. All procedures were completely endoscopic, performed under the guidance of images displayed on the monitor. At the conclusion of each procedure, transesophageal echocardiography was used to exclude prosthesis malfunction, to verify that there was no perivalvular leak and to confirm air removal.
Statistical analysis
Comparisons between the 2D and 3D group were made using Student’s t-test for continuous variables. Pearson χ2 test was used for comparisons of categorical variables. A P value less than 0.05 was considered statistically significant. All analyses were performed using the SPSS version 19.0 (SPSS Inc., United States).
Results
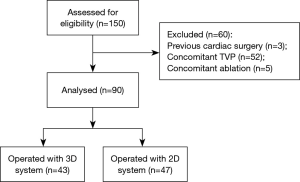
Between June 2013 to June 2016, 150 consecutive patients who underwent TEMVR were included. The analysis excluded 60 patients who failed to meet the exclusion criteria: 52 with concomitant tricuspid valve repair, 5 with concomitant ablation for atrial fibrillation, and 3 with previous cardiac surgery. For the remaining 90 patients, the 3D system was used in 43 while the 2D system was used in 47 (Figure 3).
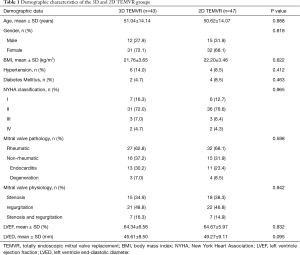
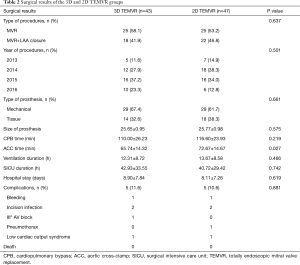
Demographic characteristics and preoperative examination results are displayed in Table 1. No significant differences were observed between the two groups. Surgical results are displayed in Table 2. There were no perioperative deaths in each group. All the procedures were performed endoscopically without conversion to middle sternotomy. Intraoperative complications including mitral prosthesis obstruction, perivalvular leak or left ventricular rupture did not occurred in both groups. No significant differences were observed between the 3D and 2D image systems with respect to type of procedure (P=0.637), year of procedure (P=0.501), postoperative duration of ventilation (P=0.466), length of SICU stay (P=0.742), or length of hospital stay (P=0.619). Major postoperative complications occurred in 10 patients (3D vs. 2D, 5 vs. 5, P=0.881, Table 2). Bleeding requiring revision occurred in 2 patients, both were well-controlled endoscopically. Postoperative low cardiac output syndrome (cardiac output index <2 L/min/m2) occurred in 2 patients, for whom intra-aortic balloon pump was used for circulatory assist.
Full table
Full table
The aortic cross clamp time in the 3D group (65.74±14.32 min) was significantly shorter than that of the 2D group (72.67±14.67 min; P=0.027). The cardiopulmonary bypass time was also shorter in the 3D group, but not significantly (110.00±26.23 vs. 116.60±23.93 min; P=0.219).
Discussion
Currently, endoscopic cardiac surgery is mainly based on 2D, 3D, and robotic image systems. The 2D image system was invented and implemented first. After over 30 years of modification and improvement, 2D is now widely used in minimally invasive cardiac surgery with excellent results. However, the lack of depth perception and spatial orientation with the 2D image system is now recognized as a major drawback (2). The robotic system provides both high-definition stereovision and precise surgical performance. The robots are competent in challenging procedures such as total endoscopic coronary artery bypass surgery (3). Nevertheless, the robotic system requires specific instruments that takes a much longer time to prepare and a prolonged learning curve for surgeons to adopt the technique (4). Most importantly, the expansion of the robotic system is substantially limited due to its extraordinary high cost, especially in developing countries (5). In comparison with the 2D system, the 3D image system provides a natural sense of depth and better hand-eye coordination. In addition, the costs for 3D and 2D surgery are similar, both of which are much lower than those of robotic surgery. Furthermore, tactile feedback is perfectly preserved in 3D surgery, another advantage over robotic surgery.
The 3D-HD video system has already been shown to be superior to the 2D-HD system in terms of shorter operative time in endoscopic surgeries, including pulmonary lobectomies (6-9) and esophagectomies (10). In the 2D images, monocular cues were used to compensate for the lack of depth perception, including motion parallax through movement of the scope, relative position, size of instruments, anatomic structures, shading of light and dark, and texture grading (11,12). The surgeons were actually performing the procedure with one eye closed, which is a clear handicap during the initial learning phase. In the 3D images, the separate input from two viewpoints allows for summation on a cortical level (13). Visual acuity improves by 10% using binocular vision (14). The use of 3D devices has already been shown in randomized studies to shorten the learning curve and to shorten task performance time for laparoscopic surgery, both for novice trainees (15) and experienced surgeons (16).
TEMVR as a single and fixed procedure was compared between two homogenous group of patients in our study: all patients were relatively young, with similar mitral valve disease etiology, operated by the same surgeon using identical surgical techniques. Even though, we still observed approximately 10% of reduction in the aortic cross-clamp time. We believed this significant improvement was mainly attributed to the change of the image system. When performing TEMVR, 3D images provided valuable depth information regarding the left atrium and left ventricle, as well providing structural details regarding the mitral valve leaflets, chordae tendineae, and papillary muscles. We felt much more confident when resecting the diseased valvular leaflets and placing sutures around the annulus under 3D image guidance. Less time was wasted with repetitive and correctional moves compare to the 2D TEMVR. In addition, the anti-fog feature of the 3D camera head definitely reduced the time for lens cleaning during surgery.
There was no significant difference between the 3D and 2D group in terms of postoperative mechanical ventilation time, length of SICU stay, length of hospital stay, or incidence of major complications. Our data suggested that 2D and 3D TEMVR are both safe procedures in the surgical treatment of mitral valvular diseases.
In spite of so many advantages, there are still much room for improvement for the current version of the 3D-HD imaging system. To some extent, the depth perception of the surgical field may be exaggerated and distorted by the 3D-HD image system. As a result, unpleasant feelings including nausea, vertigo, and visual fatigue occur occasionally. The camera head of 2D endoscope can rotate separately from the hand shank, so a 360-degree view is available. However, the camera head for the 3D endoscope has to rotated with the hand shank together. As a result, the entire image is rotated as well. It is very challenging for a surgeon to operate under a rotated view.
Study limitations
Our study is based on a single surgeon’s experience, since each surgeon has different perception and adaptability of the 3D images, individual discrepancy has to be considered. The study design is non-randomized therefore it is difficult to eliminate selection bias in the allocation of the 2D and 3D groups. Seven minutes difference in aortic cross-clamp time may not be convincing enough in favour of the 3D system. In addition, the sample size was relatively small, larger randomized control trials are necessary for further confirmation of the benefits of the 3D-HD video system.
Conclusions
The 3D-HD video system appears to be superior to the 2D system for TEMVR, with better surgical performance and similar operative safety. The 3D vision is a promising technology that is worth wide promotion in the field of cardiac surgery.
Acknowledgements
Funding: This work is supported by Science and Technology Planning Project of Guangdong Province, China (grant number: 2017B030314109) and Grant of Guangdong Provincial People’s Hospital (grant number: 2017zh06).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by our Institutional Ethics Review Board. Informed consent forms were obtained from all patients enrolled.
References
- McLachlan G. From 2D to 3D: the future of surgery? Lancet 2011;378:1368. [Crossref] [PubMed]
- Honeck P, Wendt-Nordahl G, Rassweiler J, et al. Three-dimensional laparoscopic imaging improves surgical performance on standardized ex-vivo laparoscopic tasks. J Endourol 2012;26:1085-8. [Crossref] [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Technical challenges in totally endoscopic robotic coronary artery bypass grafting. J Thorac Cardiovasc Surg 2006;131:146-53. [Crossref] [PubMed]
- Oh D S, Cho I, Karamian B, et al. Early adoption of robotic pulmonary lobectomy: feasibility and initial outcomes. Am Surg 2013;79:1075-80. [PubMed]
- Ding R, Tong X, Xu S, et al. A comparative study of Da Vinci robot system with video-assisted thoracoscopy in the surgical treatment of mediastinal lesions. Zhongguo Fei Ai Za Zhi 2014;17:557-62. [PubMed]
- Yang CL, Wang W, Mo LL, et al. Short-Term Outcome of Three-Dimensional Versus Two-Dimensional Video-Assisted Thoracic Surgery for Benign Pulmonary Diseases. Ann Thorac Surg 2016;101:1297-302. [Crossref] [PubMed]
- Bagan P, De Dominicis F, Hernigou J, et al. Complete thoracoscopic lobectomy for cancer: comparative study of three-dimensional high-definition with two-dimensional high-definition video systems dagger. Interact Cardiovasc Thorac Surg 2015;20:820-3. [Crossref] [PubMed]
- Dong S, Yang XN, Zhong WZ, et al. Comparison of three-dimensional and two-dimensional visualization in video-assisted thoracoscopic lobectomy. Thorac Cancer 2016;7:530-4. [Crossref] [PubMed]
- Jiao P, Wu QJ, Sun YG, et al. Comparative study of three-dimensional versus two-dimensional video-assisted thoracoscopic two-port lobectomy. Thorac Cancer 2017;8:3-7. [Crossref] [PubMed]
- Li Z, Li JP, Qin X, et al. Three-dimensional vs two-dimensional video assisted thoracoscopic esophagectomy for patients with esophageal cancer. World J Gastroenterol 2015;21:10675-82. [Crossref] [PubMed]
- Hanna GB, Shimi SM, Cuschieri A. Task performance in endoscopic surgery is influenced by location of the image display. Ann Surg 1998;227:481-4. [Crossref] [PubMed]
- Falk V, Mintz D, Grunenfelder J, et al. Influence of three-dimensional vision on surgical telemanipulator performance. Surg Endosc 2001;15:1282-8. [Crossref] [PubMed]
- Wenzl R, Lehner R, Vry U, et al. Three-dimensional video-endoscopy: clinical use in gynaecological laparoscopy. Lancet 1994;344:1621-2. [Crossref] [PubMed]
- Rabin J. Two eyes are better than one: binocular enhancement in the contrast domain. Ophthalmic Physiol Opt 1995;15:45-8. [Crossref] [PubMed]
- Tanagho YS, Andriole GL, Paradis AG, et al. 2D versus 3D visualization: impact on laparoscopic proficiency using the fundamentals of laparoscopic surgery skill set. J Laparoendosc Adv Surg Tech A 2012;22:865-70. [Crossref] [PubMed]
- Wilhelm D, Reiser S, Kohn N, et al. Comparative evaluation of HD 2D/3D laparoscopic monitors and benchmarking to a theoretically ideal 3D pseudodisplay: even well-experienced laparoscopists perform better with 3D. Surg Endosc 2014;28:2387-97. [Crossref] [PubMed]