
Extra-pleural pneumonectomy
Introduction
The extra-pleural pneumonectomy (EPP) is a standardised procedure of en bloc resection of the parietal and visceral pleura with the ipsilateral lung, pericardium, and hemidiaphragm (1).
In 1949, Sarot described the EPP technique for tuberculous infection resistant to collapse therapy or thoracoplasty (2) and in 1976, for the first time, Butchart et al. employed EPP malignant pleural mesothelioma (MPM) patients (3).
For many years, the EPP represented the gold standard surgical choice in MPM patients, but its clinical role has been revised after publication of the mesothelioma and radical surgery (MARS I) trial (4).
For other pleural malignancies, such as thymomas, low-grade sarcomas (5-7) this surgical procedure is accepted in many referral centres in selected cases, while it is more questionable its use in non-small cell lung cancer (NSCLC) with pleural carcinosis (8).
Nowadays, this type of surgery is always a part of a multimodality treatment program, consisting of neoadjuvant chemotherapy, hyperthermic intraoperative chemotherapy (HIOC), adjuvant chemo/radiotherapy (9-11).
Methods
The search strategy and the selection
We performed our literature search using In PubMed, OVID, Embase, and Cochrane, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews according to PRISMA protocol, from January 1985 to January 2018 (12). The words for the research were “extra-pleural pneumonectomy”, “malignant pleural mesothelioma”, “pleural malignancies”. We found 89,475 references. Data were extracted using a standard collection form. Two independent reviewers extracted information from each study including author names, year of publication, number of patients, intervention, control, outcomes, and adverse effect. Two independent reviewers and disagreements assessed discussion and consensus settled the risk of bias. By reading the titles and the abstracts, we eliminated duplicated articles, cases reports, comments, letters to the editor and publications about infective and benign pathologies (search result: 1,986 papers). In the end, the references with surgical interest for EPP operation were 68.
Results
From 68 articles, we had a total of 3,220 patients who underwent EPP for MPM (3,117 patients) or other pleural malignancies (103 patients) obtained from our selected studies All the information about type of study, number of patients, 5 years overall survival (OS), morbidity, mortality and adjuvant/neoadjuvant treatments are summarized in Table 1. Since the retrospective study may contain selection bias, we show the results from prospective studies in Table 2.
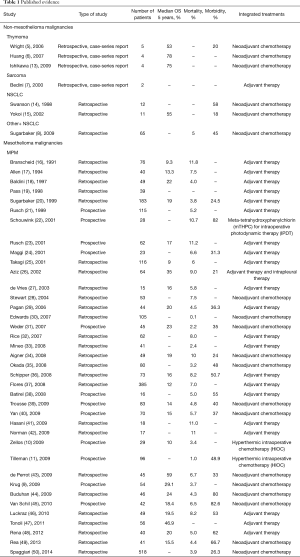
Full table
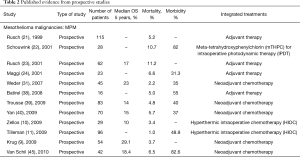
Full table
The cardio-respiratory pre-operative evaluation is necessary for all EPP patients’ selection: pulmonary function test, diffusion capacity, pulmonary scan, complete cardiological study with a stress test for inducible myocardial ischemia, echocardiogram with Doppler, pulmonary artery measurement (51).
There are general guidelines about the predictive post-pneumonectomy FEV1 tested at least 0.8 L, but in EPP there is not a cut-off value shared from all the authors; someone considered predictive post-operative FEV1 cut-off 1.2 L because of the diaphragm removal, and for more extended surgical incision, with possibility of muscles damage (52), although there are other functional evaluations for pneumonectomy according to ACCP guidelines (53).
This not standardised pre-operative evaluation makes more complicated the interpretation of the results from so many different centres, and this is true for mortality, morbidity and QoL.
The anaesthesiologic preparation for EPP includes placement of an arterial line, a central line, and an epidural catheter for perioperative regional pain control and sometimes a Swan-Ganz catheter, double-lumen endotracheal tube, a nasogastric tube (54).
The EPP is a surgical procedure performed and studied mainly in MPM patients. This tumour can be surgically treated with EPP or with pleurectomy/decortication (P/D), so it represents a comparative analysis model between two different types of surgery: lung-sparing procedure vs. more radical and aggressive surgery (43). All the most essential information about EPP, arise from MPM patients series, who have been studied with the aim to match the results of EPP vs. P/D or more recently any surgery but chemo/radiotherapy (4,55-57).
For many years, the EPP radical surgery in mesothelioma patients has been performed by many authors until multicentre randomised controlled MARS trial (4) showed no more benefit derived to MPM patients, from this vs. chemotherapy alone.
However, in both cases, the MPM staging is critical, and the PET-computed tomography is necessary for distant metastases detection but, for nodal involvement evaluation, sometimes, the endobronchial ultrasonography and or cervical mediastinoscopy can be necessary (21,58). Chest MRI is useful to evaluate for chest wall, transdiaphragmatic, or transmediastinal invasion of a tumour (59).
In other non-mesothelioma pleural malignancies, because of the smaller number of patients and heterogeneity of biological behaviours, this comparative analysis has never been performed and could never get the same statistical significance than in MPM.
The overall five years survival ranged from 0 to 78% (6) in thymoma patients and 56% (43) in MPM patients.
An extended posterolateral or lateral thoracotomy is required. The extra-pleural plane is dissected from endothoracic fascia all over the pleural space. Posteriorly the limits are the azygous vein on the right and the aorta on the left; superiorly the subclavian vessels and anteriorly the mammalian vessel and the thymic fat. The hemi-diaphragm represents the inferior limit with his pericardial and chest wall attachments. Heart (when there is an intrapericardial extension), aorta, superior and inferior vena cava, oesophagus represent the essential structures we should care to not damage, during the dissection.
The phrenic nerves and vessels can be clipped especially if the hemidiaphragm is resected. After pericardial anterior and posterior incision, the vascular, pulmonary hilum is prepared, and the vessels are sutured and divided. The mainstem bronchus is divided and closed with a heavy wire stapler, and it can be buttressed with thymic tissue or omentum mobilised on a vascularized flap. It is preferable to avoid intercostal muscle pedicle, particularly if the rib is removed. The diaphragm can be reconstructed with different mesh/patch (52). The diaphragm resection and reconstruction are a critical point in EEL. Autologous latissimus dorsi reverse or alloplastic materials can be used for diaphragmatic repair flap (60,61). The division of chest from abdomen prevents the herniation of the stomach and other abdominal organs.
Post-operative complications
The post-EPP behaves differently to post-pneumonectomy, and this must be considered in a mediastinal shift and for fluid/air balance (62).
The early identification of the most life-threatening post-operative complications such as ARDS, pericardial tamponade, cardiac herniation, pulmonary embolism, respiratory infections, respiratory failure, atrial arrhythmia, myocardial infarction described by Sugarbaker in 2004 on 328 cases (1), is necessary for keeping a low accidents rate.
MPM
The peri-operative mortality and morbidity, in mesothelioma patients, ranged from 0–11.8% (16), and from 0–82.6% (45), respectively. Already in his old article, in 1999 Sugarbaker (20), showed in MPM patients a perioperative mortality rate of 3.8% with five years OS of 15%. In MPM, Sugarbaker and Rusch (51,63) showed as a significant factor of OS staging, histology (epithelial better prognosis), integrated treatment and sex; while N2 status was a worse prognostic factor in de Perrot analysis (43) and Flores series (56,58). In 1999, it was already accepted that locally advanced T and N status, and non-epithelial histology was prognostic factors of poor prognosis (21).
There are also two studies evaluating quality of life (QoL) after EPP in MPM patients (64,65), and Cao et al. showed an improvement in QoL at three months.
The ipsilateral chest is the most common site of treatment failure after EPP-based multimodality therapy (37,47,66).
Other pleural malignancies
We have only seven studies focused on non-mesothelioma pleural malignancies. About sarcoma pleural dissemination treated with EPP, the literature is limited to rare report (7); Sugarbaker reported a median survival of 3.7 months in 10 sarcoma patients (8). Wright (5) presented five patients who underwent EPP for stage IVa thymoma, with any postoperative deaths and one major complication (tamponade requiring removal of the pericardial patch); the overall 5-year survival was 53%.
In NSCLC stage IV with pleural effusion after induction chemotherapy (67), the EPP is one possibility, but we are so far from recognizing this as a standard treatment of non-distant- metastatic pleural carcinosis form NSCLC; it remains such a questionable indication, that is inevitably needed more prospective studies. Swanson et al. (14) presented 12 NSCLC patients with malignant pleural effusion and no N2/N3 nodal or distant metastases treated between 1994 and 1997. After neoadjuvant chemotherapy, the patients, who did not have any progression, underwent EPP, with 0% mortality and 58% of morbidity.
Sugarbaker et al. (8) between 1994 and 2007 reported 28 patients N0 stage IV NSCLC for pleural effusion treated with EPP, obtaining median survival for the nine patients with N0 disease of 52 months, 14 months for the 19 for N1 and/or N2 disease, suggesting a role of EPP in survival improvement. Yokoi et al. (15) at the Tochigi Cancer Centre in Japan treated with EPP 11 patients who for NSCLC with malignant pleural effusion, including three patients with the clinical N2 disease, showing an overall 5-year survival of 54.5%, and in pathological N0–N1 disease on final patients experienced a 5-year survival of 67%.
Multimodality integrated treatments
In MPM, the most used drugs as neoadjuvant treatment were carboplatin, cyclophosphamide, and gemcitabine, more recently replaced by pemetrexed and cisplatin (58). Sugarbaker and Wolf, in their 183 MPM patient’s series, reported already many chemotherapy cycles with doxorubicin and cyclophosphamide with or without cisplatin, and later carboplatin and paclitaxel (51). Three authors analysed in MPM HIOC (10,11,68) as hyperthermic intraoperative intracavitary chemotherapy perfusion following extrapleural pneumonectomy with acceptable morbidity and mortality. Schouwink et al. (22) showed, with meta-tetrahydroxyphenylchlorin (mTHPC) for intraoperative photodynamic therapy (IPDT), good results in local control of disease in 50% of the treated cases, although much toxicity rate is procedure-correlated. Aziz et al. presented a comparative analysis between two MPM groups: a series of 51 patients who underwent to EPP, adjuvant intrapleural normothermic carboplatin, and a series of 13 patients who underwent EPP alone. The results showed a better median OS in the group that received adjuvant intrapleural and systemic chemotherapy (35 versus 13 months) (26).
Comments
We decided to review the EPP for all the thoracic malignancies not only focusing on the MPM only since we want to have a complete revision of the literature. At the moment, in sarcoma and thymoma patients there are too much few data to standardise EPP as a recognised choice treatment. In MPM patients the indications are changed and in pleural carcinosis from NSCLC stage IV, “wet stage IV NSCLC”, the chemotherapy is the gold standard.
The references selected are published between 1991 and 2014, and the perioperative management has been changed drastically. Therefore, to discuss the perioperative mortality and morbidity, the study period should be narrowed.
Also, the EPP should be always a part of a multimodality treatment program and often is the only one possibility for thoracic locally extended cancer treatment, especially in young patients, with low-grade disease, non-responsive to systemic chemotherapy.
In very selected cases and with the aim to obtain R0 margins, in deeply extended diseases through the chest wall, this resection has been extended over the EPP to thoracopleuropneumonectomy (TPP) (69) so that, with an increased number of patients we should comparatively analyse EPP vs. TPP in non-mesothelioma low grade malignancies, such as it was done with P/D vs. EPP in MPM.
In the referral centre with expertise, EPP could represent a radical procedure for pleural malignancies, especially for rare diseases, in selected advanced stages, always sharing the indications in the multidisciplinary meeting.
The timing of surgery integrated approach, the identification of disease worth of this type of surgery, and the functional cardio-pulmonary cut-off and limits for correctly undergoing to EEP represent the most critical questions about this significant surgery we should try to standardise, although it could be useful just in a limited number of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46. [Crossref] [PubMed]
- Sarot IA. Extrapleural pneumonectomy and pleurectomy in pulmonary tuberculosis. Thorax 1949;4:173-223. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg 2006;82:1234-9. [Crossref] [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007;134:1477-83. [Crossref] [PubMed]
- Bedini AV, Tavecchio L, Delledonne V. Extrapleural pneumonectomy for sarcomas report of two cases. Tumori 2000;86:422-3. [Crossref] [PubMed]
- Sugarbaker DJ, Tilleman TR, Swanson SJ, et al. The role of extrapleural pneumonectomy in the management of pleural cancers. J Clin Oncol 2009;27:7577.
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Zellos L, Richards WG, Capalbo L, et al. A phase I study of extrapleural pneumonectomy and intracavitary intraoperative hyperthermic cisplatin with amifostine cytoprotection for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2009;137:453-8. [Crossref] [PubMed]
- Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405-11. [Crossref] [PubMed]
- Wieseler B, McGauran N. Reporting a Systematic Review. Chest 2010;137:1240-6. [Crossref] [PubMed]
- Ishikawa Y, Matsuguma H, Nakahara R, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg 2009;88:952-7. [Crossref] [PubMed]
- Swanson SJ, Jaklitsch MT, Mentzer SJ, et al. Induction chemotherapy, surgical resection and radiotherapy in patients with malignant pleural effusion, mediastinoscopy negative (stage IIIB) non-small cell lung cancer. American Association for Thoracic Surgery 78th Annual Meeting Abstract Book 1998. Boston, May 3-6, 1998.
- Yokoi K, Matsuguma H, Anraku M. Extrapleural pneumonectomy for lung cancer with carcinomatous pleuritis. J Thorac Cardiovasc Surg 2002;123:184-5. [Crossref] [PubMed]
- Branscheid D, Krysa S, Bauer E, et al. Diagnostic and therapeutic strategy in malignant pleural mesothelioma. Eur J Cardiothorac Surg 1991;5:466-72. [Crossref] [PubMed]
- Allen KB, Faber LP, Warren WH. Malignant pleural mesothelioma. Extrapleural pneumonectomy and pleurectomy. Chest Surg Clin N Am 1994;4:113-26. [PubMed]
- Baldini EH, Recht A, Strauss GM, et al. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 1997;63:334-8. [Crossref] [PubMed]
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1998;115:310-7; discussion 317-8. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63. [Crossref] [PubMed]
- Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999;68:1799-804. [Crossref] [PubMed]
- Schouwink H, Rutgers ET, van der Sijp J, et al. Intraoperative photodynamic therapy after pleuropneumonectomy in patients with malignant pleural mesothelioma: dose finding and toxicity results. Chest 2001;120:1167-74. [Crossref] [PubMed]
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95. [Crossref] [PubMed]
- Maggi G, Casadio C, Cianci R, et al. Trimodality management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2001;19:346-50. [Crossref] [PubMed]
- Takagi K, Tsuchiya R, Watanabe Y. Surgical approach to pleural diffuse mesothelioma in Japan. Lung Cancer 2001;31:57-65. [Crossref] [PubMed]
- Aziz T, Jilaihawi A, Prakash D. The management of malignant pleural mesothelioma; single centre experience in 10 years. Eur J Cardiothorac Surg 2002;22:298-305. [Crossref] [PubMed]
- de Vries WJ, Long MA. Treatment of mesothelioma in Bloemfontein, South Africa. Eur J Cardiothorac Surg 2003;24:434-40. [Crossref] [PubMed]
- Stewart DJ, Martin-Ucar A, Pilling JE, et al. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg 2004;78:245-52. [Crossref] [PubMed]
- Pagan V, Ceron L, Paccagnella A, et al. 5-year prospective results of trimodality treatment for malignant pleural mesothelioma. J Cardiovasc Surg (Torino) 2006;47:595-601. [PubMed]
- Edwards JG, Martin-Ucar AE, Stewart DJ, et al. Right extrapleural pneumonectomy for malignant mesothelioma via median sternotomy or thoracotomy? Short- and long-term results. Eur J Cardiothorac Surg 2007;31:759-64. [Crossref] [PubMed]
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202. [Crossref] [PubMed]
- Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685-92; discussion 1692-3.
- Mineo TC, Ambrogi V, Pompeo E, et al. The value of occult disease in resection margin and lymph node after extrapleural pneumonectomy for malignant mesothelioma. Ann Thorac Surg 2008;85:1740-46. [Crossref] [PubMed]
- Aigner C, Hoda MA, Lang G, et al. Outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;34:204-7. [Crossref] [PubMed]
- Okada M, Mimura T, Ohbayashi C, et al. Radical surgery for malignant pleural mesothelioma: results and prognosis. Interact Cardiovasc Thorac Surg 2008;7:102-6. [Crossref] [PubMed]
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257-64. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortications in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504. [Crossref] [PubMed]
- Trousse DS, Avaro JP, D’Journo XB, et al. Is malignant pleural mesothelioma a surgical disease? A review of 83 consecutive extra-pleural pneumonectomies. Eur J Cardiothorac Surg 2009;36:759-63. [Crossref] [PubMed]
- Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 2009;138:619-24. [Crossref] [PubMed]
- Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010-6. [Crossref] [PubMed]
- Norman PH, Thall PF, Purugganan RV, et al. A possible association between aprotinin and improved survival after radical surgery for mesothelioma. Cancer 2009;115:833-41. [Crossref] [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [Crossref] [PubMed]
- Buduhan G, Menon S, Aye R, et al. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 2009;88:870-5. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Luckraz H, Rahman M, Patel N, et al. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:552-6. [Crossref] [PubMed]
- Tonoli S, Vitali P, Scotti V, et al. Adjuvant radiotherapy after extrapleural pneumonectomy for mesothelioma. Prospective analysis of a multi-institutional series. Radiother Oncol 2011;101:311-5. [Crossref] [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: A harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): feasibility and results. Lung Cancer 2007;57:89-95. [Crossref] [PubMed]
- Spaggiari L, Marulli G, Bovolato P, et al. Extrapleural pneumonectomy for malignant mesothelioma: an Italian multicenter retrospective study. Ann Thorac Surg 2014;97:1859-65. [Crossref] [PubMed]
- Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med 2010;4:363-72. [Crossref] [PubMed]
- Wolf AS, Daniel J, Sugarbaker DJ. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:132-48. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Hartigan PM, Ng JM. Anesthetic strategies for patients undergoing extrapleural pneumonectomy. Thorac Surg Clin 2004;14:575-83. [Crossref] [PubMed]
- Federico R, Adolfo F, Giuseppe M, et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer 2013;13:22. [Crossref] [PubMed]
- Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. [Crossref] [PubMed]
- Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating surgery for mesothelioma after radiation therapy: the “SMART” approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. [Crossref] [PubMed]
- Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: implications for revision of the American Joint Committee on Cancer Staging System. J Thorac Cardiovasc Surg 2008;136:605-610. [Crossref] [PubMed]
- Patz EF Jr, Shaffer K, Piwnica-Worms DR, et al. Malignant pleural mesothelioma: value of CT and MR imaging in predicting resectability. AJR Am J Roentgenol 1992;159:961-6. [Crossref] [PubMed]
- Rolli L, Leuzzi G, Girotti P, et al. Permeable Nonabsorbable Mesh for Total Diaphragmatic Replacement in Extended Pneumonectomy. Ann Thorac Surg 2017;104:e105-7. [Crossref] [PubMed]
- Bedini AV, Andreani SM, Muscolino G. Latissimus dorsi reverse flap to substitute the diaphragm after extrapleural pneumonectomy. Ann Thorac Surg 2000;69:986-8. [Crossref] [PubMed]
- Wolf AS, Jacobson FL, Tilleman TR, et al. Managing the pneumonectomy space after extrapleural pneumonectomy: postoperative intrathoracic pressure monitoring. Eur J Cardiothorac Surg 2010;37:770-5. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Ambrogi V, Mineo D, Gatti A, et al. Symptomatic and quality of life changes after extrapleural pneumonectomy for malignant pleural mesothelioma. J Surg Oncol 2009;100:199-204. [Crossref] [PubMed]
- Baldini EH, Richards WG, Gill RR, et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2015;149:1374-81. [Crossref] [PubMed]
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9. [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Pastorino U, Duranti L, Scanagatta P, et al. Thoracopleuropneumonectomy with riblike reconstruction for recurrent thoracic sarcomas. Ann Surg Oncol. 2014;21:1610-5. [Crossref] [PubMed]


