
Management of calcified hilar lymph nodes during thoracoscopic lobectomies: avoidance of conversions
Introduction
Within the last three decades, the use of video-assisted thoracoscopic surgery (VATS) has evolved from initial observation and biopsy to surgical resection and reconstruction (1-5). In 1992, the first VATS lobectomy incorporating anatomic hilar dissection was performed. As the technology has continued to improve, it is now applied in more surgical fields, such as lobar sleeve resection, pulmonary artery repair, and even tracheal and carinal reconstruction (6-8). The utilization of VATS has also increased due to its attractiveness as a minimally invasive modality with significantly reduced postoperative complications and length of hospital stay (9-12).
Despite these improvements, however, conversions to open thoracotomies occasionally occur, with an incidence rate ranging from 2% to 20% (13). The most common reasons for this include unexpected vessel injuries and uncontrollable bleeding, intense hilar adhesions, and extensive disease progression to the hilum (14). Mal-manipulation of the hilar lymph nodes frequently leads to arterial injury and massive bleeding, which in most circumstances requires open thoracotomy for bleeding control. In a case series of 232 VATS lobectomies, 40% (6/15) of the conversions occurred after impairment of the pulmonary artery and difficult lymph node management (15).
In this paper, we introduce a novel surgical technique for the safe management of calcified hilar lymph nodes. Below, we describe the method in detail, as well as the results of its clinical application to a consecutive case series.
Methods
The Shanghai Pulmonary Hospital institutional ethics committee approved this retrospective study (IRB No. K18-103).
Patients
The 12 patients in this study underwent an initial case evaluation for the appropriateness of conducting the VATS operation. When lung cancer of a radiologically solid type was strongly suspected, remote metastasis was excluded by the results of a whole-body bone scan, abdominal ultrasound examination, brain MRI, and enhanced chest computed tomography (CT) scan. Alternatively, a positron emission tomography (PET)-CT was performed. In the presence of N2 disease, 2–4 cycles of new-adjuvant chemotherapy were generally suggested, followed by re-evaluation for surgery.
A total of 12 patients were enrolled in this study upon discovery of calcified hilar lymph nodes during the operation, and the pulmonary artery was determined to be not mobilizable by the conventional technique. To proceed with the anatomical lobectomy, the new technique was then initiated.
Radiological evaluation of hilar lymph nodes
Although CT films were regularly evaluated and pre-operative consultations occurred among senior surgeons in our institute, the characteristic findings of hilar nodal calcifications were generally omitted due to the technical difficulty of their evaluation. As such, their presence was more often an incidental finding during an operation and was reliant on the operative experience of the respective surgeon. We postoperatively reviewed radiological images in this study and then produced a retrospective summarization.
Operative manipulations
The patients were intubated with a double lumen tube after general anesthesia and then placed in the lateral position. Generally, one surgical port is preferred by our institute at the 4th or 5th intercostal space along the ante-axillary line. After entering the chest cavity, we exposed and then dissected the target hilum for the lobectomy. The conventional flowchart indicates “one-direction” dissection, which starts by stapling the pulmonary vein. Next, using energy instruments, either the lobar bronchus or the branches of the lobar pulmonary artery are dissected and well exposed, which is then closed with a stapler. Fissures are usually stapled last before removal of the specimen.
In the presence of calcified hilar lymph nodes, dissection of the target artery or bronchus is challenging. It is technically impossible to find adipose space between the nodes and the artery, which will result in a torn vessel if the surgeon attempts to free the vessel using blunt forceps. When attempting to open the vascular sheath, dissection may also tear the vessels due to the tenderness of the artery wall, which is a consequence of nodal compression. It is also impossible to expose the back side of vessels during this step. Seemingly, the only recourse is to control the trunk of the main artery. Arterial injury always occurs at the deepest corner of vessel exposure, which makes it technically impossible to repair in the absence of adequate bleeding control.
The new “scissor-first” technique
The essence of this novel operative technique is its focus on using sharp dissection to find the right extra-bronchial space, while keeping the artery intact with the calcified nodes. Before employing this technique, the lymph nodes must first be biopsied and sent for frozen section. With a negative result, the hilum can then be anatomically dissected as follows.
Figures 1,2 show the details of this technique. When using scissors, adhesiolysis is practically safe along the bronchus. The use of an electrode or energy harmony device is generally not recommended, but if applied it must be handled with extreme caution. Generally, there are two dissection techniques. The first is to suture across the nodes and then tie the vessel branches (Figure 1). The underlying requisite is only that the artery branch is small (e.g., the ascending branch of the upper lobe) and well exposed by more than 2/3 of its circumference. This procedure can be safely and repeatedly adjusted if the needle accidentally goes straight across the vessel on the first attempt, which is usually indicated by blood oozing from the needle holes. After tying the artery, scissors are used to free the vessel from the bronchus along with the lymph nodes. The lymph node, in this step, is gradually transected until the scissors meet the bronchus. Then, the scissors should be heading downwards along the bronchus wall, meeting the level of bronchial bifurcates.
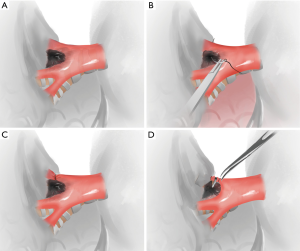
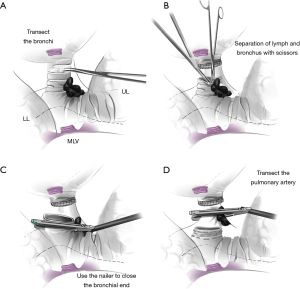
The second method is applied when dissecting the lobar bronchus, or when the artery branch is not adequately exposed (>1/3 of its circumference remains unexposed) (Figure 2). Firstly, the segmental bronchi were transected where caution must be taken to secure an adequate tumor margin. Then, the space between the bronchus and node could be clearly observed. The nodes provided a heavy barrier thus preventing the artery from incidental injury. The scissors, then, go downward to the lobar origin until the pulmonary artery trunk is completely freed. Since the calcified node is now left on the vessel branch, blue staples are preferred for transecting the pulmonary artery. After removal of the specimen, the stump of the lobar bronchus can then be easily closed either with a stapler or absorbable suture, depending on the length of the stump (Figure 2).
The remaining surgical manipulations, including lymph node dissection or sampling, bronchial stump reinforcement if necessary, and chest tube placement, are performed in the routine manner.
Results
General information
Of the 12 enrolled patient cases, there were seven males and five females, with an average age of 72.5 [66–80] years. Of these, there were five right-upper lobectomies, three right-middle lobectomies, three right-lower lobectomies, and one left-lower lobectomy. All were lung cancers at a clinically early stage, as determined by pre-operative examination. No contra-indications were revealed. No patients received induction treatments (Table 1).
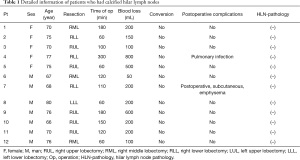
Full table
Radiological readings
A retrospective review of the hilar lymph nodes on CT images proved unremarkable. High-density calcification was confirmed only occasionally in two cases. More commonly, the hilar nodes manifested as irregular, granular, or nodular shadows with very close adherence to the lobar bronchus over a wide range of contact. The fat interspace between the nodes and pulmonary artery was undiscernible in all cases. We noted no obvious nodal bulging or compressions onto the artery and no heterogeneous density changes in the nodes.
Surgical outcomes
Ten patients were provided with uniportal access to the chest cavity and the other two had two-port incisions, due to their extensive pleural adhesions. All the target hilar lymph nodes were negative by frozen section, and based on these results, we used the proposed technique without resection of the calcified nodes. In all 12 cases, there were no difficulties in handling the veins; all the lobar veins were stapled in the routine manner. The lobar bronchus was stapled in 10 cases, and were closed with 4-0 absorbable sutures in the other two cases. All the operations proceeded generally smoothly with an average operative time of 125 [60–300] minutes. The mean blood loss was 275 [50–800] mL.
Few post-operative complications occurred; there was only a pulmonary infection and a subcutaneous emphysema, which were resolved with routine antibiotics application. The average hospitalization time was 5.7 [3–14] days. Final pathology results indicated early-stage adenocarcinomas in all 12 cases (4 at pT1bN0M0, 3 at pT1bN0M0 pT1cN0M0, 2 at pT2aN0M0, and 3 at pT2bN0M0).
Discussion
Calcified hilar lymph nodes, which are also referred to as “nail lymph nodes”, have been a critical factor in conversion to open thoracic surgery due to its characteristic features of hard, strong adhesions closely clinging to the blood vessel, which prevent their separation (13-16). The most common reasons for their formation include: senile tuberculosis, recurrent nodal inflammation subsequent to pulmonary fungus infection, chronic bronchitis, bronchiectasis, and chronic obstructive pulmonary disease (16-20). Smoking and industrial waste gas, which are also associated with chronic inflammation, are aggravating factors (21,22).
The presence of calcified hilar lymph nodes makes thoracoscopic hilar management a huge challenge. In 2013, Hanna reported that the conversion rate reached 25–40% because of hilar lymph node calcification and adhesion, which is associated with significantly prolonged operation and hospitalization times (23). Samson also concluded that the calcification score of hilar lymph nodes was the main forecast factor of transitioning to thoracotomy (24). The underlying reason for this challenge was the encircling of the bronchi or artery without any loose interspace, which frequently results in tearing of the pulmonary artery and massive bleeding (25). Therefore, the routine dissection of the bronchus or artery often fails in the presence of benign sticky or inflammatory hilar nodes. In contrast with hilar tumorous invasions, en-bloc resection and reconstruction are necessary in this case, without requiring dissection of the lobar hilum.
There are also some other technical manipulation options, such as the “vascular, anatomy, lymph node and technical (VALT) Open” technique proposed by Gazala and associates (13). This technique is used to repair ruptured blood vessels and avoid conversions and addresses the timing of conversions. Another method is to control the main trunk beforehand and free the artery branch using sharp dissection. Mechanical tear of the artery may then be repaired using 5-0 non-absorbable sutures. This technique, however, requires additional mobilization of the artery trunk and distal branch, and therefore increases the technical complexity as well as the operative risk. The blocking device in the thorax may also impair later surgical thoracoscopic manipulations, especially those with a one-port incision.
There were also some limitations in this article. Firstly, when we found calcified lymph nodes, partial sampling was first operated, and only all negative lymph node metastasis can we use this technique. However, negative biopsy does not fully stand for negative lymph node metastasis. It still exists potential risks of false negative lymph node metastasis. Secondly, this technique relies on experiences of surgeons. Thoracotomy approach would be recommended.
In this paper, for the first time, we introduced a practical, safe, and efficient hilum management technique involving no vessel blockage or plastic reconstruction. The key maneuver is to locate the right interspace between the bronchial wall and lymph nodes. Therefore, integration of the artery is well maintained because the nodes remain intact along with the artery.
Acknowledgements
Funding: This study was supported by award (16CR3018A) from the Shanghai Hospital Development Center.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Shanghai Pulmonary Hospital institutional ethics committee approved this retrospective study (IRB No. K18-103).
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Landreneau RJ, Hazelrigg SR, Ferson PF, et al. Thoracoscopic resection of 85 pulmonary lesions. Ann Thorac Surg 1992;54:415-9; discussion 419-20. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE, et al. One hundred consecutive patients undergoing video-assisted thoracic operations. Ann Thorac Surg 1992;54:421-6. [Crossref] [PubMed]
- Lewis RJ, Sisler GE, Caccavale RJ. Imaged thoracic lobectomy: Should it be done? Ann Thorac Surg 1992;54:80-3. [Crossref] [PubMed]
- Stanley DG. Thoracoscopic lobectomy. J Tenn Med Assoc 1992;85:463-4. [PubMed]
- Moon DH, Lee JM, Jeon JH, et al. Clinical outcomes of video-assisted thoracoscopic surgery esophagectomy for esophageal cancer: A propensity score-matched analysis. J Thorac Dis 2017;9:3005-12. [Crossref] [PubMed]
- Li J, Liu H, Liu J, et al. Challenges in complex video-assisted thoracoscopic surgery and spontaneous respiration video-assisted thoracoscopic surgery procedures. J Vis Surg 2017;3:31. [Crossref] [PubMed]
- Mun M, Nakao M, Matsuura Y, et al. Video-assisted thoracoscopic surgery lobectomy for non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2018;66:626-31. [Crossref] [PubMed]
- McKenna RJ Jr, Fischel RJ, Wolf R, et al. Video-assisted thoracic surgery (VATS) lobectomy for bronchogenic carcinoma. Semin Thorac Cardiovasc Surg 1998;10:321-5. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Kim HK, Sung HK, Lee HJ, et al. The feasibility of a two-incision video-assisted thoracoscopic lobectomy. J Cardiothorac Surg 2013;8:88. [Crossref] [PubMed]
- Gazala S, Hunt I, Valji A, et al. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:962-4. [Crossref] [PubMed]
- Scarci M, Gonzalez-Rivas D, Schmidt J, et al. Management of Intraoperative Difficulties During Uniportal Video-Assisted Thoracoscopic Surgery. Thorac Surg Clin 2017;27:339-46. [Crossref] [PubMed]
- Augustin F, Maier HT, Weissenbacher A, et al. Causes, predictors and consequences of conversion from vats to open lung lobectomy. Surg Endosc 2016;30:2415-21. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Survival of patients with unsuspected N2 (stage IIIa) nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:362-6; discussion 366-7. [Crossref] [PubMed]
- Rueth NM, Andrade RS. Is vats lobectomy better: Perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim J, et al. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:1288-93. [Crossref] [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: Single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [Crossref] [PubMed]
- Villamizar NR, Darrabie M, Hanna J, et al. Impact of T status and N status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg 2013;145:514-20; discussion 520-1. [Crossref] [PubMed]
- Shen JF, Zhang XX, Li SB, et al. Complete video-assisted thoracoscopic surgery for pulmonary sequestration. J Thorac Dis 2013;5:31-5. [PubMed]
- Zhou ZL, Zhao H, Li Y, et al. Completely thoracoscopic lobectomy for the surgical management of bronchiectasis. Chin Med J (Engl) 2013;126:875-8. [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [PubMed]
- Samson P, Guitron J, Reed MF, et al. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: A retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg 2013;145:1512-8. [Crossref] [PubMed]
- Guido Guerrero W, Gonzalez-Rivas D, Hernandez Arenas LA, et al. Techniques and difficulties dealing with hilar and interlobar benign lymphadenopathy in uniportal VATS. J Vis Surg 2016;2:23. [PubMed]


