
Use of staplers and adverse events in thoracic surgery
One of the key requirements for the use of staplers in surgery in general is the need to find a balance between adequate tissue compression time and the risk of increased tissue tearing and excessive tensile strength (1). In thoracic surgery, some specificities of the lung tissue, whose peripheral and central areas vary greatly in content of solid (bronchi) and more plastic elements (alveoli), directly influence the stapling process. In addition, increases in lung tissue thickness are typical for lung cancer and pulmonary fibrosis. These changes, together with lung emphysema and tuberculosis, affect the efficiency of staplers as well.
The reports about the benefits of staplers followed a certain time trend. The initial reports referred mostly to advantages and disadvantages of manual vs. mechanical suture of the bronchus in open surgery. After the onset of lung volume reduction surgery (LVRS), most of the data related to the role of the stapler line reinforcement. With the widespread use of video-assisted thoracoscopic surgery (VATS), the focus switched towards adverse events (AEs) during vascular stapling. The widespread use of VATS brought another type of problems, not strictly related to staplers, but more to difficulties in stapler positioning related to the limited space.
What are the differences in the use of staplers?
Nowadays, in addition to initially used “L”-shaped TA bronchial and vascular staplers, endo-staplers are being increasingly used during open surgery as well, mostly to reduce the operation time and improve surgical comfort. One major difference relates the disease stage—unlike the early stage lung cancer, being the usual indication for the use of endo-vascular staplers in VATS surgery, the use of vascular TA staplers in open surgery frequently represents the only possible option in presence of locally advanced tumours, like for example in presence of short main pulmonary artery stump or invaded extrapericardial part of the pulmonary vein. In this situation the most proximally invaded vessel is stapled the last, after having dealt with other vessels, so that the distal end can be freely cut without the unplanned blood loss. The second difference relates to the use of bronchial staplers—in open surgery TA staplers are usually used, whilst “V”-shaped endo-stapler jaws are most frequently used in VATS procedures.
Several reasons for stapler-related problems in VATS vs. open surgery are commonly reported: (I) problems in selection between the 3.5 and 4.5 mm staple height due to difficulty in assessment of the thickness and resistance of lung tissue in the limited space setting; (II) the relatively small jaw opening of endo-stapler can make loading of the tissue difficult, associated with friction and subsequent tearing of the tissue; (III) difficulty in precisely positioning the stapler tip may result in excessive tissue traction (2).
AEs related to vascular stapling
Vascular stapler AEs, always requiring a prompt action, have one additional, “hidden” aspect that is not to be neglected: the existing literature suggests the direct relationship between perioperative blood transfusion and worse overall survival [risk ratio (RR) 1.25, P<0.001], and worse recurrence-free survival (RR 1.42, P<0.001), underlying the importance of approaches and technologies associated with lower risks of perioperative blood transfusion (3).
In most of the series, the intraoperative AE rate is low. Rarely, some AEs occur after the operation as well. The reported rate of AEs as a direct consequence of vascular stapling in series with 842–2,548 vascular divisions is 0.1–0.27% (4,5). When extended to all types of vascular injuries during VATS surgery in series including 414–3,076 patients, the AE rate reaches 2.2–4.1% (6). Somewhat higher AE rates are the consequence of the type of vascular injuries that were reported as AEs, like 8.2% incidence of vascular injuries in 73 patients undergoing VATS lobectomy (7). These data included vascular injuries during vascular dissection and stapler positioning as well.
Several types of vascular AEs are reported, like stapling failure, oozing, laceration of the adjacent vessels, technical vascular injury at insertion or rupture of the stapling stamp. In addition to above mentioned causes of stapler related problems, tissue fragility, stapler rocking during stapling, stapler-tissue thickness mismatch and technical failure were reported as causes of AEs as well.
As for prevention of AEs during vascular stapling, in order to deal with tissue fragility and stapler-tissue thickness mismatch as the possible causes of AE, cartridge with longer staples are suggested as a possible way to reduce such AEs. On the other hand, to decrease oozing, development of cartridge with shorter staples has also been suggested (8). Since powered vascular staplers have been launched (9), their use has been increasingly reported as useful in AE prevention, like in a retrospective study more than 700 hospitals in the USA or a recent multicenter study conducted in China (10). Care should be taken if this type of stapler is used for smaller caliber vessels, because of the unavoidable vibration movement of the device at the end of the cutting phase. In this situation some surgeons prefer a manual endo stapler. Recently, new V-shaped compression staplers with dull side edges to prevent laceration of the adjacent vasculature have become available. The curved tip of the anvil and a rubber flexible attachment make the insertion to the hilar structures much easier using such devices.
The prerequisite for vascular stapling AE prevention is to get as good overview as possible before dividing vascular structures, either by some further opening of partially fused fissures or by applying a “tunnel” technique (11,12). The awareness of possible anatomical bronchovascular variations is equally important. One should be proactive in a way to search for the “missing” or “remaining” artery or to facilitate the orientation by identifying the correct bronchus.
Dealing with AE during vascular stapling is not a subject of this text. However, it is worth mentioning that a prompt reaction is mandatory, but not necessarily in form of immediate conversion. In some situations, a vessel compression during a couple of minutes suffices, giving enough time for subsequent decision about definitive solution (Figure 1).
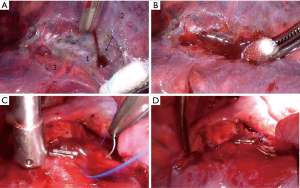
As the literature data in this field are still scarce, results of rare experimental studies are of great benefit, despite some limitations of experimental studies. As early as 60 years ago experiments with stapling devices of this era demonstrated that “The defined values of isolated organ walls are not so reliable for estimation of suturing gap range in instruments, as they do not include the tissue turgor that depends on blood filling, tissue fluid pressure, and other factors.” (13). The study of Shimizu et al. published in this issue (14), reporting results of animal experiment on pig cardiopulmonary blocks, combined with retrospective review of clinical videos of 263 patients, overcome some of these limitations. This study showed that the pulmonary vessel stapling causes stress due to twisting and lifting that decreased stump durability. Avoiding such stress was suggested as important for AE prevention.
Bronchial stapling AEs
Despite all advances in thoracic surgery, the development of a bronchopleural fistula (BPF), especially after pneumonectomy, persists as a major postoperative complication. Although the reported incidence of BPF seems to be lower after stapling (2.0–5.2%) vs. hand suturing (6.6–18.2%) (15), there is still no agreement among on which technique is better, because a low percentage of fistulas was also reported after manual closure (16). Currently, mechanical staplers seem to be the ones preferred by most surgeons, depending on countries und technical facilities.
The advantages of using staplers are minimized contamination of operation area and markedly reduced time required for closure; disadvantages become evident when dealing with thickened airways or in close proximity to a tumor. In this situation, eventual AEs, mostly in form of immediate or early staple-line failure, represent the consequence of inappropriate intraoperative assessment—stapling vs. manual suture/some of the bronchoplastic procedures, rather than being directly related to stapling device. It is also clear that the oncological radicality remains questionable in case of bronchial stapling in presence of tumours with proximal endobronchial spread, because manual cutting and closure gives the possibility for eventual bronchoplastic procedure in case of positive frozen section result.
The most frequently reported risk factors are the same for both techniques: cachexia, destructive changes in lungs, anemia, hyperglycemia, chronic obstructive pulmonary disease, preoperative chemo- or radiotherapies applied for lung cancer, hypoalbuminemia, previous steroid therapy, the American Society of Anesthesiologists (ASA) class ≥3, long-lasting postoperative intubation because of respiratory dysfunction (17). Sometimes the evidence may be confusing, in a way that some reports did not confirm that preoperative chemo- or chemoradiotherapy increase BPF rate (18).
As for the BPF prevention, independently of the bronchial closure technique, the need to keep the stump not too long and circulation preserved as much as possible, remains out of debate. Based on the fistula location in the stump’s center after main bronchus stapling in their patients, some authors hypothesized that the cause was increased tension in the membranous part of the bronchus due to compression by the stapler. They subjected patients undergoing pneumonectomy to the modified conventional procedure, by folding both sides of the cartilaginous wall while stapling, without subsequent stump coverage. None of the patients developed a fistula although a larger study is required to confirm these results (15).
As one of technical innovations designed to decrease the BPF rate in open surgery are curved staplers, that have a greater surface area than linear staplers (19). This device exerts less force on the tissue at an equal compressive force per unit of area, distributing the compression over a wider area, in the same time enabling “cutting” operation. Moreover, compared with the 15 staples delivered by the TA-45, the curved stapler delivers a total of 46 staples. It also has a longer handle, and this facilitates access to the rib cage.
Independently of the used bronchial closure technique, bronchial stump protection remains the standard procedure for AE prevention. There is a broad consensus that bronchial stump protection with vascularised flaps plays an important role in the prevention of complications, especially in patients undergoing a pneumonectomy after chemo or chemo/radiation therapy and especially for right-sided operations. The most frequently reported flaps are intercostals muscle, pericardium, pericardial fat, diaphragm, omentum, azygos vein or latissimus dorsi.
The technique of harvesting and efficacy of the intercostals flap has been sufficiently documented quite a long ago. The key-point of this quite simple technique is to avoid placement of a rib spreader before harvesting the full flap thickness and preservation of the periosteum. Recently, thoracoscopic and robotic preparation of this kind of flap have been reported as well (20). No major complications have been reported, except pedicle ossification, usually without major clinical implications.
Owing to consistent anatomy of thoracodorsal vessels, it is possible to split the latissimus dorsi muscle into separate units, so that the use of lateral split muscle flap has also been described (21). The harvested muscle flap is rotated anteriorly and is passed through the second or third intercostals space into the pleural cavity. This method is in some centers preferred to frequently used serratus transposition, because the problem of wing scapula can be avoided.
Diaphragm flap seems to be more frequently used than reported in the literature. Rare reports are in favour of its use as efficient for the bronchial stump reinforcement after pneumonectomy.
Pericardial fat pad graft is a very suitable solution, but not always available, especially in thin individuals or after previous lung resection. The viability of this flap is based on thin pericardial branches of the internal mammary artery.
The omentum has been reported as very useful for the bronchial suture protection, with a very low rate of failure, like in 35 patients with a high risk of fistula after right pneumonectomy and in two patients with an acute bronchial fistula, with only one patient with treatment failure (22).
The main disadvantage of the omental transposition, the extension of the operation into the abdomen, usually in patients not fit for major surgery, can be overcome by recently proposed transdiaphragmatic harvesting, thus avoiding a laparotomy.
Were experimental studies of help in this field, like for vascular stapling?
It has been demonstrated that the sutured bronchial stump heals primarily through secondary closure. The analysis of the bronchial stump one week after right upper lobectomy in dogs showed that the incidence of adhesion formation between the surrounding tissues, the thickness of granulation tissue over the stump and its vessel density were significantly reduced in the stapler group in comparison to the suture group (23). Another study showed that mechanical suture of the bronchial stump, submitted to pressure immediately after closure, is more resistant than manual suture in dogs submitted to pneumonectomy (24). Similar results were confirmed using sheep as an animal model, but in another experiment the same research team also demonstrated that the higher stability and therefore the increased resistance to pressure of the mechanical suture disappears once the bronchial stump has healed, becoming equal to that of the manual suture (25).
Lung tissue staplers
The use of staplers in presence of poorly defined fissures represented a major advance in open surgery several decades ago, in comparison with lung clamping and suture, being the only possible technique before the stapler appearance. The efficiency of these staplers is well established in this situation, independently of the used type—linear staplers for open surgery or endo-staplers. The eventual AEs are more related to surgical technique and skills, rather than being related to the device itself. Related to this type of staplers, the ability of linear staplers for open surgery to include more lung tissue in a single firing sequence is counterweighted by its positioning difficulties through the narrow “tunnel” created between the vessels in case of poorly defined fissures. Currently, with the availability of 60-mm Endo GIA staplers, enabling stapling through quite a thick lung tissue, they are being used more and more frequently during the open surgery as well.
In LVRS surgery, several techniques have been used to prevent and minimize air leaks, including buttress materials such as bovine pericardium, polytetrafluoroethylene (PTFE), Teflon, polyglycolic acid and gel foam. The technique, either with or without staple line reinforcement is well established. A new staple line reinforcement material made from porcine small intestinal submucosa (SIS) was suggested as well. Unlike synthetic or cross-linked meshes, products made from SIS are engineered to facilitate tissue remodeling while being slowly incorporated into the body. In a study on 25 patients undergoing LVRS with the stapler line reinforcement with an autologous fibrin sealant, the air-leak severity score, prolonged air leaks and the mean duration of chest tube drainage were significantly lower in the main than the control group (26).
Conclusions
The available literature of stapling AEs in thoracic surgery is still scarce. As their treatment usually represents a medical urgency with unpredictable outcome, AEs prevention is of utmost importance. Experimental studies are of great help in order to achieve this goal.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Collopy BT. Colorectal anastomotic leak rates are measures of technical skill in surgery. ANZ J Surg 2001;71:508-10. [Crossref] [PubMed]
- Gossot D, Merlusca G, Tudor A, et al. Pitfalls related to the use of endostaplers during video-assisted thoracic surgery. Surg Endosc 2009;23:189-92. [Crossref] [PubMed]
- Luan H, Ye F, Wu L, et al. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg 2014;14:34. [Crossref] [PubMed]
- Asamura H, Suzuki K, Kondo H, et al. Mechanical vascular division in lung resection. Eur J Cardiothorac Surg 2002;21:879-82. [Crossref] [PubMed]
- Szwerc MF, Landreneau RJ, Santos RS, et al. Minithoracotomy combined with mechanically stapled bronchial and vascular ligation for anatomical lung resection. Ann Thorac Surg 2004;77:1904-9; discussion 1909-10.
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary Resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Kawachi R, Tsukada H, Nakazato Y, et al. Morbidity in video-assisted thoracoscopic lobectomy for clinical stage I non-small cell lung cancer: is VATS lobectomy really safe? Thorac Cardiovasc Surg 2009;57:156-9. [Crossref] [PubMed]
- Yano M, Takao M, Fujinaga T, et al. Adverse events of pulmonary vascular stapling in thoracic surgery. Interact Cardiovasc Thorac Surg 2013;17:280-4. [Crossref] [PubMed]
- Echelon Flex Powered Vascular Stapler. Ethicon. Cincinnati. Available online: http://www.ethicon.com/healthcareprofessionals/products/staplers/endocutters/echelonfle-powered-vascular-stapler#!science-and-technology. Accessed 15 Nov 2017.
- Qiu B, Yan W, Chen K, et al. A multi-center evaluation of a powered surgical stapler in video-assisted thoracoscopic lung resection procedures in China. J Thorac Dis 2016;8:1007-13. [Crossref] [PubMed]
- Nomori H, Ohtsuka T, Horio H, et al. Thoracoscopic lobectomy for Lung cancer with a largely fused fissure. Chest 2003;123:619-22. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a ‘fissure first, hilum last’ approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Astafiev G. Investigation of processes relating to tissue compression in suturing and stapling apparatus. Surgical Staplers 1967;7:22-31. (Chirurgicheskiye Shivayushiye Apparaty).
- Shimizu N, Tanaka Y, Okamoto T, et al. How to prevent adverse events of vascular stapling in thoracic surgery: recommendations based on a clinical and experimental study. J Thorac Dis 2018;10:6466-71. [Crossref] [PubMed]
- Aoki T, Ozeki Y, Watanabe M, et al. Cartilage folding method for main bronchial stapling. Ann Thorac Surg 1998;65:1800-1. [Crossref] [PubMed]
- Hubaut JJ, Baron O, Al Habash O, et al. Closure of the bronchial stump by manual suture and incidence of bronchopleural fistula in a series of 209 pneumonectomies for lung cancer. Eur J Cardiothorac Surg 1999;16:418-23. [Crossref] [PubMed]
- Cicėnas S, Jackevičius A, Aškinis R, et al. Methods of main bronchus stump closure and incidence of bronchopleural fistula after pneumonectomies for lung cancer (a retrospective single center review). Acta Medica Lituanica 2013;20:183-9.
- Refai M, Brunelli A, Rocco G, et al. Does induction treatment increase the risk of morbidity and mortality after pneumonectomy? A multicentre case-matched analysis. Eur J Cardiothorac Surg 2010;37:535-9. [Crossref] [PubMed]
- Sardelli P, Barrettara B, Cisternino ML, et al. Curved cutter stapler for the application of bronchial sutures in anatomic pulmonary resections: the clinical experience of 139 cases. Eur J Cardiothorac Surg 2012;41:653-6. [Crossref] [PubMed]
- Sagawa M, Sugita M, Takeda Y, et al. Video-assisted bronchial stump reinforcement with an intercostal muscle flap. Ann Thorac Surg 2004;78:2165-6. [Crossref] [PubMed]
- Terzi A, Luzzi L, Campione A, et al. The split latissimus dorsi muscle flap to protect a bronchial stump at risk of bronchial insufficiency. Ann Thorac Surg 2009;87:329-30. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Andreetti C, et al. Transdiaphragmatic harvesting of the omentum through thoracotomy for bronchial stump reinforcement. Ann Thorac Surg 2009;88:212-5. [Crossref] [PubMed]
- Izumi Y, Kawamura M, Gika M, et al. Granulation tissue formation at the bronchial stump is reduced after stapler closure in comparison to suture closure in dogs. Interact CardioVasc Thorac Surg 2010;10:356-9. [Crossref] [PubMed]
- Bof AM, Rapoport A, Paulo DN, et al. Comparative study of the resistance of manual and mechanical sutures in the bronchial stump of dogs submitted to left pneumonectomy. J Bras Pneumol 2007;33:141-7. [Crossref] [PubMed]
- Ludwig C, Hoffarth U, Haberstroh J, et al. Resistance to pressure of the stump after mechanical stapling or manual suture. An experimental study on sheep main bronchus. Eur J Cardiothorac Surg 2005;27:693-6. [Crossref] [PubMed]
- Moser C, Opitz I, Zhai W, et al. Autologous fibrin sealant reduces the incidence of prolonged air leak and duration of chest tube drainage after lung volume reduction surgery: a prospective randomized blinded study. J Thorac Cardiovasc Surg 2008;136:843-9. [Crossref] [PubMed]


