
Intramuscular stimulation as a novel alternative method of pain management after thoracic surgery
Introduction
Postoperative pain as a result of thoracotomy is a serious problem for thoracic surgeons; it is not only associated with postoperative respiratory complications, but also with the induction of fear and psychological distress in patients undergoing thoracic surgery (1). To minimize postoperative pain, thoracic surgeons, in efforts to lessen the degree of incision, have developed mini-thoracotomy, video-assisted thoracoscopic surgery (VATS), and more recently, single-port VATS to lessen the degree of incision (2).
To further manage and reduce postoperative pain in patients undergoing VATS, various pain-management methods have been introduced. Among these, epidural patient-controlled analgesia (PCA) and intravenous (IV)-PCA are most widely used. However, these methods involve continuous injection of narcotics, increasing the risk of developing side effects, including nausea, vomiting, dizziness, constipation, urinary retention, and other systemic-related side effects. To mitigate these side effects, techniques using a catheter, such as continuous-slow infusion of local anesthetics into the paravertebral or direct wound space, have recently been established (3-5). This paravertebral technique, according to Raveglia and colleagues (6), has superior results compared with the PCA methods in thoracotomy patients; however, it has not been fully evaluated with respect to VATS.
Single-port VATS, as the name suggests, utilizes only one incision site (7). Therefore, it is believed to cause much less postoperative pain then the other methods of thoracic surgery; however, to the best of our knowledge, postoperative pain management after single port VATS has not been well developed to date. Therefore, this prospective, randomized study compared two postoperative pain management methods—IV-PCA and electrical twitch-obtaining intramuscular stimulation (ETOIMS)—in pneumothorax patients undergoing single-port VATS. We determined whether ETOIMS can be a viable alternative to IV-PCA in the postoperative acute phase for those with pneumothorax undergoing single-port VATS.
Methods
Patient entry and randomization
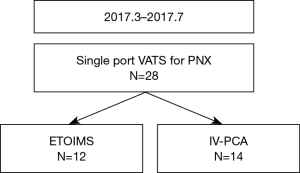
This study was approved by the Institutional Review Board of Gangnam Severance Hospital (3-2017-0016). Patients who were initially treated for primary spontaneous pneumothorax and underwent single-port VATS for recurrence between March and July 2017 were enrolled in this preliminary prospective randomized trial. The study was designed as a superiority test. The sample size was determined statistically using Cohen’s effect size. All patients prior to participation were provided with an explanation about the study, and written informed consent was obtained from all participants. In the operating room using a table of random numbers generated by a computer, participants were randomly allocated to one of the two group: the ETOIMS group or the IV-PCA group. Twenty-eight patients were initially enrolled (Figure 1). Two patients in the ETOIMS group were excluded due to malfunctioning of ETOIMS device; hence, a total of 26 patients were enrolled for final analysis (ETOIMS group, n=12; IV-PCA group, n=14).
Anesthesia and IV-PCA
All patients received a premedication of midazolam (0.02–0.04 mg/kg), followed by IV propofol (1.5–2.0 mg/kg) at induction. Then, after infusion of remifentanil (0.5–20.0 µg/kg/min) and confirmation of the loss of consciousness, rocuronium bromide (0.8 mg/kg) was administered; after 90 seconds, upon muscle relaxation, intubation was performed. An IV-PCA device, a continuous-infusion type with elastomeric pump, was connected to patients in the IV-PCA group immediately after the operation. IV-PCA bottle, which holds 100 mL of the solution, containing fentanyl 10 µg/mL and ramosetron 0.3 µg was delivered (Accufuser Plus, Woo Young Medical, Chungbuk, Korea) with a basal infusion rate of 2 mL/h, bolus dose of 0.5 mL, and a lockout period of 15 minutes.
Surgical procedure
All surgical procedures were performed by a single surgeon with patients in lateral decubitus position under general anesthesia using double-lumen endotracheal tube for one-lung ventilation. Bullae and blebs were verified using a high-resolution computed tomography (HRCT) before performing surgery. For patients requiring closed thoracostomy, 12F trocar catheter (Argyl, suture rib trocar catheter; Coviden, Mansfield, MA, USA) was inserted at the 5th intercostal space (ICS) on the anterior axillary line (AAL) before surgery to reduce symptoms. A minimal, transverse incision was made for this to allow for a 20-mm elongated incision at the time of surgery. For all single-port VATS procedures, a 20-mm skin incision was made at the 5th ICS on AAL using a wound protector. A 5-mm 30° video thoracoscope was used to carefully observe the apex and superior segment of the lower lobe for any bullae or blebs. Upon detection, complete wedge resection was performed using endoscopic stapler (EndoGIA articulating stapler; Covidien). We reinforced the stapling lines by covering them with absorbable polyglycolic acid (PGA) (NEOVEIL®; Gunze, Ayabe, Japan) and fibrin glue. After the procedure, a single 20 F chest tube was placed in the thoracic cavity through a single incision site. The chest tube was removed when there was no air leakage and the amount of fluid drainage was less than 150 mL/day.
ETOIMS procedure
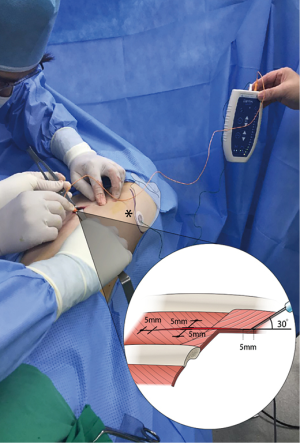
All ETOIMS procedures were performed using the Dantec ClavisTM device. After completing the main operation, but before closing and repairing the wound, we visually confirmed the intercostal muscle and performed needle stimulation, using a monopolar needle with a diameter of 250 µm. As a reference electrode, a surface electrode was attached onto the skin at the ipsilateral scapular inferior angle. After insertion of an aseptic monopolar needle into the intercostal muscles, we started a stimulation of unipolar negative waves with 2 mA of intensity, 0.2 ms of pulse duration, and 1 Hz of frequency. Stimulation was carried out for 8–10 seconds at each site, inducing intercostal muscle twitching. The monopolar needle was inserted with a depth of 5 mm, at a 30-degree inclination into the four sites within 5 mm of the upper, lower, right, and left incision lines (Figure 2).
Postoperative pain control
An IV-PCA device, with elastomeric pump and continuous infusion, was connected to patients in the IV-PCA group immediately after the operation. In the event of inadequate postoperative pain relief, additional IV analgesics (pethidine 25 mg or ketorolac 30 mg) were administered in both groups. Ibuprofen arginine (368.9 mg; Carol-FTM) was provided, orally, every 6 hours to all patients who were able to swallow.
Assessment of postoperative pain
All patients, after extubation and gaining consciousness, were transferred to the post-anesthesia care unit (PACU). After about an hour or so in the PACU, they were transferred to the general ward. The pain score was assessed by a single pain clinic nurse at 5 time points: immediately after the surgery in PACU, 4 and 8 hours after the transfer to the general ward, as well as 1 and 2 days after the operation. Pain sensitivity was estimated using the visual analogue scale (VAS). For comparison between the two groups, the mean pain score was used if it was assessed more than once in a given day. The pain score assessed in the PACU was analyzed separately to illustrate the immediate postoperative pain. A VAS score of 0–3 was considered as effective pain management; 4–6 was considered as moderate pain and patients were allowed additional IV analgesics upon request; and a score of 7 or greater was considered as intolerable pain and patients were provided with additional analgesia.
Statistical analysis
Descriptive statistics were used to compare the variables between the groups, using χ2 test or Fisher exact test for categorical variables and the Student’s t-test for continuous variables. A linear model for repeated measures covariance pattern model with unstructured covariance with participants was used. Two fixed effects were included: one addressing the between group effect (group ETOIMS and group IV-PCA) and one addressing the within time [level: PACU, VAS-4 h, VAS-8 h, VAS-postoperative day (POD) 1, VAS-POD 2]. Possible differences between the groups across time were analyzed according to group x time interactions. The interaction between the group and time was tested at a significance level of 0.05. Hypothesis testing was two sided at a significance level of 0.05. Sample size for mixed model (repeated measures ANOVA) was calculated according to our primary endpoints. We considered two group and five-time repetitions to detect differences of VAS. We calculated that a sample size of 22 participants was sufficient for detecting an effective value of 0.25 (medium effect) and determining the correlation among repeated measures of 0.5 at a significance level of 0.05 (two-sided) with 80% power. All statistical analyses were performed using SAS V.9.3 (SAS Institute Inc., Cary, North Carolina, USA).
Results
A total of 26 patients were analyzed—12 patients in the ETOIMS group and 14 patients in the IV-PCA group. The baseline characteristics are shown in Table 1. The mean age of patients in the ETOIMS group was 19.1±4.8 years and that in the IV-PCA group was 18.8±5.0 years (P=0.879). There were no significant differences in sex, body-mass index, operation site, operation time, chest tube indwelling time, and hospital stay between the two groups.

Full table
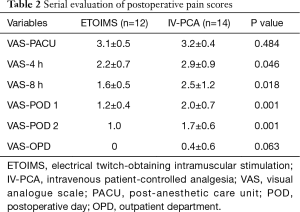
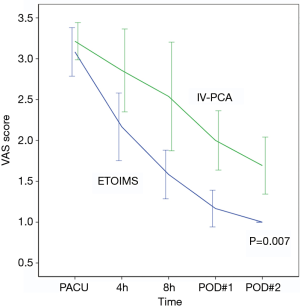
Serial evaluation of postoperative pain score immediately after surgery in PACU showed no significant difference between the two groups, with 3.1±0.5 in the ETOIMS group and 3.2±0.4 in the IV-PCA group. However, there was a significant difference between the two groups at time points after PACU, at postoperative hour 4, postoperative hour 8, POD 1, and POD 2 (Table 2). Using a linear mixed model, we determined that there was a clear difference in the average change of VAS score over time between the two groups (P=0.007), as shown in Figure 3. As a result of comparing between the two groups at each time point, we found that there was a significant difference at postoperative hour 8 (P=0.015), POD 1 (P=0.001), and POD 2 (P=0.001). Moreover, when comparing the VAS score within each group against the baseline score immediately following surgery at PACU, there was a significant difference at every time point—postoperative hour 4 (P=0.001), postoperative hour 8 (P=0.001), POD 1 (P<0.001), and POD 2 (P<0.001)—in the ETOIMS group; in the IV-PCA group, however, there was a significant difference starting at postoperative hour 8 (P=0.019), POD 1 (P<0.001), and POD 2 (P<0.001).
Full table
In the analysis of analgesic requirement during the postoperative period, we observed a significant difference with respect to the number of patients receiving one or more administrations of IV-analgesia between the two groups; 2 (16.7%) in the ETOIMS group and 8 (57.1%) in the IV-PCA group (P=0.034) (Table 3). Moreover, there was a significant difference in the number of patients who developed analgesic-induced side effects between the two groups; there was one patient (8.3%) with side effects in the ETOIMS group, while there were 7 patients (50.0%) with side effects in the IV-PCA group (P=0.022) (Table 4).
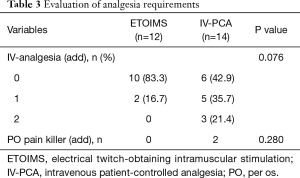
Full table
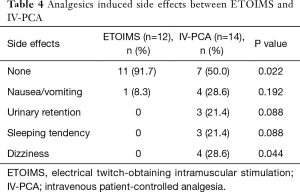
Full table
Discussion
This preliminary prospective randomized study compared the efficacy between ETOIMS and IV-PCA as a method of postoperative pain management for pneumothorax patients undergoing single-port VATS. We found that in the acute-postoperative phase, starting from postoperative hour 4, the ETOIMS group showed a statistically significant reduction in the VAS score. Moreover, this group also required less additional analgesia (P=0.034) with less analgesia-induced side effects (P=0.022).
Postoperative pain is usually due to direct surgical injury to tissues, which is often worsened by tension, cough, exercise, abdominal distension, and etc. (1). Consequently, patients, out for fear, tend to avoid exercising and coughing, as well as show a tendency to breath in a shallow and rapid manner, resulting in increased muscle contraction or tension that leads to reduced overall vital capacity (4,6). Hence, reducing fear of coughing and deep breathing is important for lung function recovery after pulmonary resection. In addition, it may be important to encourage patients to exercise to reduce postoperative complications, including hypoxia (8).
To minimize postoperative pain—in other word, to reduce direct tissue injury during surgery—thoracic surgeons have been increasingly focused on minimally invasive surgical techniques like VATS (9). In efforts to further minimize surgical tissue damage, surgeons have reduced the number of ports used to perform VATS, from multi-port VATS to single-port VATS (7,10). Moreover, many pain management methods—epidural PCA, IV-PCA, local bupivacaine injection, and etc.—have been researched (5).
Among these pain management methods, epidural PCA and IV-PCA are considered to be the two main methods to manage pain after VATS (11,12). However, the use of epidural PCA remains controversial for patients receiving VATS because it has generally been reserved for those undergoing more painful procedures, like thoracotomy. Kim et al. evaluated the efficacy of epidural PCA compared with IV-PCA in patients undergoing VATS lobectomy and concluded that there was no significant difference between the two methods of pain management, as shown by pain score, analgesic requirements, pulmonary function, satisfaction score, and incidence of side effects (13). They suggested that IV-PCA is a suitable alternative for epidural PCA inpatients receiving VATS lobectomy. In another study, it was revealed that epidural PCA is associated with potential risks, including dural perforation, infection, bleeding, hypotension, and urinary retention (14). Furthermore, epidural PCA, compared with IV-PCA, is more time-consuming and costly, as it requires an additional pain-team (15).
In our institution, we routinely use IV-PCA as the preferred pain management method for VATS. Nonetheless, IV-PCA is not without its flaws; it has frequently been accompanied with side effects, including nausea, vomiting, dizziness, and sleeping tendency, which has resulted in efforts to find an alternative (5).
Among the many factors that contribute to postoperative pain from thoracic surgery, we focused on intercostal muscle contraction (16). Intercostal muscles are comprised of short-muscle fibers; and once shortened from surgery, it is difficult to stretch back by itself since it is restricted between narrow ICS which is hard to stretch by exercise, requiring physical therapy and/or medication (17). Intramuscular stimulation (IMS) is an effective treatment for myofascial pain syndrome (18,19). It has been widely known to relax the muscles and relieve muscle pain by stimulating various trigger points. There were two case reports regarding patients with complaints about chronic chest wall pain secondary to herpes zoster infection, where pain relief was achieved immediately following myofascial trigger point injection in the intercostal muscle (20,21). Simons and Travell hypothesized that after the damage of the endplate by muscle trauma, overload, or strain, excessive acetylcholine release would create a myofascial trigger point (22). Under the hypothesis that the incised muscle would be hyper-contracted, with sequential development of myofascial trigger points, we attempted to utilize this ETOIMS technique to relax the intercostal muscle. Although the pathophysiology of myofascial pain syndrome and the mechanism of myofascial trigger point injection remain unclear, Gunn’s model, which insists on super sensitivity created in the innervated structure after peripheral neuropathy, has been widely adopted in most previous studies; the key mechanism of pain relief is presumed to be the desensitization of the nociceptive pathway (20,21,23,24).
Compared with the IV-PCA group, the ETOIMS group achieved pain relief significantly faster during the acute phase of postoperative pain, which began at postoperative hour 4. Moreover, we also observed that pain decreased continuously throughout POD 1 and 2. The results of our study indicate that ETOIMS may be a more effective method overall in mitigating postoperative pain than IV-PCA. The pain ratings in the PACU were not different between the groups. This could be contributed to the excessive trunk movement—while moving to the cart from the operation bed, and to the PACU bed from the cart—and aggravation of the intercostal muscle or suture line of the skin. Therefore, since resting in bed could alleviate this acute pain, the VAS score at postoperative hour 4 can be anticipated to be less compared with the first postoperative assessment.
Limitations
To the best of our knowledge, this is the first study to successfully use ETOIMS for pain management in single-port VATS. Although we were able to obtain good results for ETOIMS when compared with IV-PCA, there are some limitations. First, the operation time was relatively short; given that the majority of our patients receiving single-port VATS had pneumothorax, only simple-wedge resection was performed. Second, we measured the degree of pain using VAS, which is not a very objective measurement. However, to mitigate this limitation, the VAS score was measured only by one nurse from the department of anesthesia. Finally, we did not perform a double-blind test, and thus, our study may be limiting in showing the degree of pain according to pain location.
Conclusions
In conclusion, ETOIMS appears to be a safe, effective, simple, and economical alternative to IV-PCA, with comparable results, for pain-management after single-port VATS. A future study on ETOIMS is still warranted to better understand the mechanism ETOIMS with respect to pain management.
Acknowledgements
The authors would like to thank Dong-Su Jang, MFA, (Medical Illustrator) for his help with the illustrations.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Gangnam Severance Hospital (3-2017-0016) and written informed consent was obtained from all participants.
References
- Soto RG, Fu ES. Acute pain management for patients undergoing thoracotomy. Ann Thorac Surg 2003;75:1349-57. [Crossref] [PubMed]
- Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg 2013;96:977-82. [Crossref] [PubMed]
- Hsieh MJ, Wang KC, Liu HP, et al. Management of acute postoperative pain with continuous intercostal nerve block after single port video-assisted thoracoscopic anatomic resection. J Thorac Dis 2016;8:3563-71. [Crossref] [PubMed]
- Mehran RJ, Martin LW, Baker CM, et al. Pain Management in an Enhanced Recovery Pathway After Thoracic Surgical Procedures. Ann Thorac Surg 2016;102:e595-6. [Crossref] [PubMed]
- Yang HC, Lee JY, Ahn S, et al. Pain control of thoracoscopic major pulmonary resection: is pre-emptive local bupivacaine injection able to replace the intravenous patient controlled analgesia? J Thorac Dis 2015;7:1960-9. [PubMed]
- Raveglia F, Rizzi A, Leporati A, et al. Analgesia in patients undergoing thoracotomy: epidural versus paravertebral technique. A randomized, double-blind, prospective study. J Thorac Cardiovasc Surg 2014;147:469-73. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Reeve J, Denehy L, Stiller K. The physiotherapy management of patients undergoing thoracic surgery: a survey of current practice in Australia and New Zealand. Physiother Res Int 2007;12:59-71. [Crossref] [PubMed]
- Passera E, Rocco G. From full thoracotomy to uniportal video-assisted thoracic surgery: lessons learned. J Vis Surg 2017;3:36. [Crossref] [PubMed]
- Kim MS, Yang HC, Bae MK, et al. Single-Port Video-Assisted Thoracic Surgery for Secondary Spontaneous Pneumothorax: Preliminary Results. Korean J Thorac Cardiovasc Surg 2015;48:387-92. [Crossref] [PubMed]
- Wang X, Wang K, Wang B, et al. Effect of Oxycodone Combined With Dexmedetomidine for Intravenous Patient-Controlled Analgesia After Video-Assisted Thoracoscopic Lobectomy. J Cardiothorac Vasc Anesth 2016;30:1015-21. [Crossref] [PubMed]
- Yoshioka M, Mori T, Kobayashi H, et al. The efficacy of epidural analgesia after video-assisted thoracoscopic surgery: a randomized control study. Ann Thorac Cardiovasc Surg 2006;12:313-8. [PubMed]
- Kim JA, Kim TH, Yang M, et al. Is intravenous patient controlled analgesia enough for pain control in patients who underwent thoracoscopy? J Korean Med Sci 2009;24:930-5. [Crossref] [PubMed]
- Leung JM. Role of epidural anesthesia and analgesia on postoperative patient outcome. Anesthesiology 1995;83:1132. [Crossref] [PubMed]
- Luketich JD, Land SR, Sullivan EA, et al. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg 2005;79:1845-9; discussion 1849-50.
- Zhang R, Schwabe K, Kruger M, et al. Electro-physiological evidence of intercostal nerve injury after thoracotomy: an experimental study in a sheep model. J Thorac Dis 2017;9:2461-5. [Crossref] [PubMed]
- Mizuno M, Secher NH, Saltin B. Fibre types, capillary supply and enzyme activities in human intercostal muscles. Clin Physiol 1985;5:121-35. [Crossref] [PubMed]
- Fogelman Y, Kent J. Efficacy of dry needling for treatment of myofascial pain syndrome. J Back Musculoskelet Rehabil 2015;28:173-9. [Crossref] [PubMed]
- Ratmansky M, Minerbi A, Kalichman L, et al. Position Statement of the Israeli Society for Musculoskeletal Medicine on Intramuscular Stimulation for Myofascial Pain Syndrome-A Delphi Process. Pain Pract 2017;17:438-46. [Crossref] [PubMed]
- Chen SM, Chen JT, Kuan TS, et al. Myofascial trigger points in intercostal muscles secondary to herpes zoster infection of the intercostal nerve. Arch Phys Med Rehabil 1998;79:336-8. [Crossref] [PubMed]
- Rainey CE. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: a case report. Int J Sports Phys Ther 2013;8:145-61. [PubMed]
- Simons DG, Travell J. Myofascial trigger points, a possible explanation. Pain 1981;10:106-9. [PubMed]
- Dommerholt J. Dry needling - peripheral and central considerations. J Man Manip Ther 2011;19:223-7. [Crossref] [PubMed]
- Hong CZ, Simons DG. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil 1998;79:863-72. [Crossref] [PubMed]


