D-dimer value in the diagnosis of pulmonary embolism—may it exclude only?
Introduction
PE usually results from an occlusion of the pulmonary artery or its branches by a thromboembolus (1). PE is usually one of clinical manifestations of venous thromboembolism disease (VTE) and is the third (after ischemic heart disease and cerebrovascular disease) most frequent cardiovascular disease with an incidence of 1–2% in the general population and 12–20% among hospitalized patients (2-4). Approximately 60% of all VTE cases are found in hospitalized patients. Among those, 5–10% suffer from PE (2,5,6). Clinicians are concerned about PE, because of its high risk of mortality (7-10).
If left untreated (most often because undiagnosed), acute PE has a lethality high as 59%, whereas adequately treated acute PE leads to death in only 7% of cases (11). The gold standard for the diagnosis of PE is computed tomographic pulmonary angiography (CTPA) (12). However, CTPA has certain disadvantages such as radiation exposure, the need for contrast medium administration, which may cause renal failure and the high cost of the test (13). Therefore, less invasive and less expensive diagnostic tools are desirable. One of currently unresolved question is whether plasma D-dimer assays may be considered as reliable test both for excluding PE and for screening for patients at the highest risk. Plasma D-dimers are cross-linked fibrin derivatives produced when fibrin is degraded by plasmin. Elevated D-dimer levels are found in many conditions that lead to activation of blood coagulation and fibrin formation (14). Since the 1980’s. researchers have been trying to create algorithms useful for ruling out PE with plasma D-dimer assays (15-26). In almost all trials the cutoff point was established at the level of 500 ng/mL, but it was helpful only to rule out PE in patients with low and moderate clinical probability of PE. Thereafter plasma D-dimer assays have been studied in various aspects connected with VTE: the correlation of D-dimer levels with short-term or long-term mortality (27), the severity of the course of the disease (28), predicting disease relapse after stopping anticoagulant therapy (29), and correlation with radiological severity of PE (30). In addition, two studies reported the presence of correlation between increased D-dimer levels and the likelihood of PE diagnosis by CTPA (31,32). The aim of our study is to determine the practical plasma D-dimer level above which the risk of PE is sufficiently high that CTPA is mandatory.
Methods
Study population and data acquisition
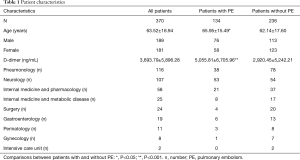
We retrospectively analyzed medical history notes of patients at University Teaching Hospital (SP CSK im. Kornela Gibińskiego in Katowice, Poland). In the hospital database from January 2009 through December 2013 we selected all patients with both a D-dimer blood level test and CTPA performed. Our inclusion criteria fulfilled 370 patients from nine wards of the hospital (Table 1). We then divided the sample into two groups: patients with PE confirmed by CTPA and those without such a diagnosis.
Full table
Plasma D-dimer assay
Plasma D-dimer levels were measured used automated quantitative latex-based, immuno-agglutination assay (HemosIL D-Dimer HS 500) (33). We included D-dimer results obtained within 48 hours before CTPA was performed. Positive test result was defined as a D-dimer level of >500 ng/mL. A normal D-dimer range was defined as <500 ng/mL.
CTPA
PE had been diagnosed by CTPA in the presence of filling defects in pulmonary arteries or images suggesting pulmonary microembolism interpreted by certified experienced radiologists.
Statistical analysis
Analysis was performed using Statistica (http://www.statistica.com/) software version 10. Receiver operating characteristic (ROC) curve were built to determine the best cutoff value for the diagnosis of PE. We first included all records and second excluded patients from surgical wards. In addition, ROC curves were built for patients aged >65 and ≤65 with and without the history of neoplasm according to the most important risk factors of thromboembolism. Because of the retrospective design of the study important clinical characteristics such as neoplasm type were not recorded. Youden’s index was calculated (YI = sensitivity + specificity – 1) for each coordinate point of the ROC curve to determine the cut-off value with the maximum sensitivity and specificity.
Results
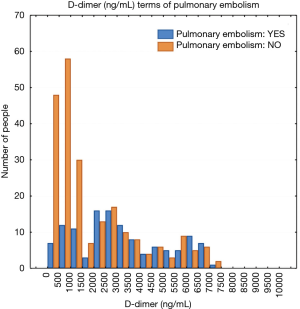
The study population included 189 men and 181 women. A total of 134 patients (36.2%) were diagnosed with PE and 236 patients (63.8%) were negative for PE. The mean plasma D-dimer level in the group with confirmed PE (5,056 ng/mL) was significantly higher than in the group of patients without PE diagnosis (2,920 ng/mL); P<0.05. The baseline characteristics of the study population are shown in Table 1. The relative frequency PE diagnosis by D-dimer range is shown in Figure 1.
Patients with positive D-dimer test
The construction of the ROC curve allowed us to determine the best cut-off point. Having D-dimer values above 2,152 ng/mL significantly increased the likelihood of a PE diagnosis [area under curve (AUC) of 0.69; 95% CI, 0.64–0.74; P<0.05] (Figure 2). Building a second ROC curve excluding patients from surgical wards (surgery, gynecology, perinatology, intensive care unit) provided similar results (the best cutoff point was 2,152 ng/mL with an AUC of 0.71; 95% CI, 0.65–0.77; P<0.05).
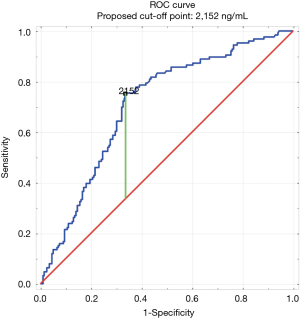
The sensitivity, specificity, PPV and negative predictive value (NPV) were calculated for different values of D-dimer concentration. For the best cutoff point (2,152 ng/mL) determined in this study, the specificity was increased from 20.3% to 62.7% (in comparison to the results for the 500 ng/mL cutoff typically used), whereas its sensitivity was at 75.4%. Also, the PPV was raised by 13.1% (from 40.3% to 53.4%), while the NPV decreased only by 5.6% (from 87.3% to 81.8%).
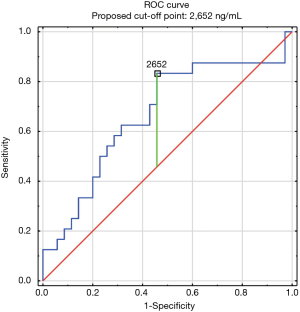
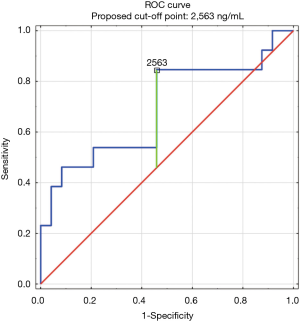
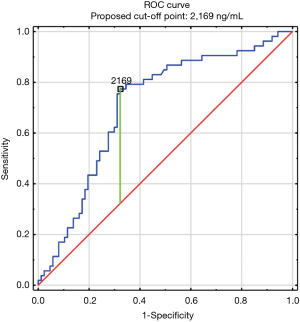
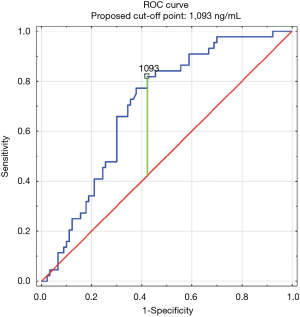
The construction of another ROC curves allowed us to determine the best cut-off points of D-dimer levels in four group samples divided according to the patients age (>65 or ≤65) years of age and the history of neoplasm. In our study, 96 patients (25.9%) had malignancy. Patients with the history of neoplasm and aged >65 had significantly increased likelihood of PE diagnosis at the D-dimer level of 2,652 ng/mL with an AUC of 0.67; 95% CI, 0.52–0.81; P<0.05 (Figure 3), whereas in patients aged ≤65 the best cut-off point was 2,563 ng/mL with an AUC of 0.69; 95% CI, 0.5–0.86; P=0.05 (Figure 4). Patients who did not suffer from neoplasm and were more than 65 years old had D-dimer cut-off point determined at the level of 2,169 ng/mL with an AUC of 0.71; 95% CI, 0.62–0.79; P<0.05 (Figure 5), whereas in patients aged ≤65 the best cut-off point was 1,093 ng/mL with an AUC of 0.7; 95% CI, 0.61–0.79; P<0.05 (Figure 6).
We also assessed the accuracy of D-dimer test in excluding PE in patients with malignancy—sensitivity was 94.6%, specificity was 6.8%, PPV 38.9% and NPV 66.7%.
Patients with negative D-dimer test
In our study, 55 patients had negative D-dimer result (under 500 ng/mL) and 7 (12.7%) of them had PE confirmed by CTPA, of which 2 (28.6%) had subsegmental PE. We also checked why CTPA was performed despite normal D-dimer concentrations in these patients. Of the 55 patients, 48 (87.27%) had extended diagnostics because they had worrying clinical symptoms and/or PE risk factors, 2 (3.6%) had pneumonia after ineffective course of antibiotics, 2 (3.6%) had higher D-dimer test levels before they were admitted to the hospital, 2 (3.6%) had CTPA performed to exclude other diseases (e.g., vascular malformations) and 1 (1.8%) could not be explained.
Discussion
The most important result of our study is the finding that highly elevated plasma D-dimer values (in our laboratory—2,152 ng/mL) are associated with significantly higher risk of PE. Our value of 2,152 ng/mL is approximately four times higher than the normal plasma D-dimer cut-off value. This finding has aroused researchers’ interest in investigating the best D-dimer cut-off level for diagnostics of PE. One of the studies based on ROC curves, determined the best cut-off level to be 830 ng/mL (AUC 0.762; 95% CI, 0.653–0.850; P<0.05), which is 1.5 times higher than the normal D-dimer concentration for that laboratory (500 ng/mL) (34). In another study, the best cut-off level based on ROC curve was determined to be 900 ng/mL (AUC 0.76; 95% CI, 0.69–0.82; P<0.001), which also was 1.5 times higher than the normal D-dimer value (580 ng/mL for that laboratory) (31). Analysis of the ROC curve in our study showed that patients with D-dimer values >2,152 ng/mL have a 69% increased risk of developing PE. In comparison, the studies previously cited indicated that cut-off levels of 830 ng/mL or 900 ng/mL have ~76% increased risk of developing PE (31,34). However, these studies had relatively small study populations and only few patients with confirmed PE. The first study had only 58 patients with PE (31) and the second had only 40 (34), whereas in our study 134 patients had confirmed PE. In addition, we analyzed medical histories of patients from nine different wards of the hospital, including internal medicine wards and surgical wards, whereas the previous studies analyzed only patients from the department of internal diseases (34) and the emergency department (31). The data on specificity and sensitivity in our study are in keeping with the conclusions of a systematic review of the literature: more sensitive is the assay less specific it becomes (35). There are large differences in proposed cut-off levels proposed for PE 1,5 times in the cited studies (31,33) which may result in a significant number of unnecessary CTPA. On the other hand, our results indicate that some patients with lower plasma D-dimer concentrations have acute PE. Therefore, prospective large scale, multicenter study should be conducted to obtain the best cut-off level.
According to the latest [2014] guidelines on the diagnosis and management of acute pulmonary embolism (PE), the PPV of elevated D-dimer levels is low and D-dimer testing is not useful for confirmation of PE and elevated D-dimer concentration has prognostic value only for short-term mortality (36). Our findings indicate otherwise. D-dimer levels may have important prognostic value in the diagnosis of PE therefore patients with low clinical probability of PE and D-dimer concentration four times exceeding normal value should be assessed carefully and considered for CTPA.
It is well known that plasma D-dimer levels may be elevated by many clinical conditions, such as postoperatively and during pregnancy (37). Therefore, we decided to exclude such cases and examine only non-surgical patients. By building another ROC curve with the exclusion of surgical patients (Surgery, Gynecology, Perinatology, and ICU), we arrived at the same cut-off point (2,152 ng/mL), with an AUC of 0.7 which indicates a comparable risk of developing PE as found using the full sample. This is the proof that our cut-off point isn’t overestimated because of other, D-dimers related conditions.
After analyzing other PE risk factors, we have determined whether the presence of two other important risk factors from Revised Geneva Score alter the cut-off points at which the likelihood of PE rises (12). Patients with an history of neoplasm had a significantly increased D-dimer cut-off level, than patients without such history regardless of their age. At the opposite, age was a significant factor in the patients without history of neoplasm with a significantly increased D-dimer cut-off level in patients >65 years old compared with those ≤65 years old. The age-adjusted D-dimer cut off point is already a well-established method of ruling out PE (38), but we propose that a better way to determine the best cut-off point above which significantly increases the likelihood of PE diagnosis is to accept the patients age as in Revised Geneva Score compared with using age-adjusted D-dimer cut-off levels. To the best of our knowledge our paper seems to be the first to describe the likelihood of PE in such groups of patients. There were only reports about importance of those two risk factors in ruling out the PE, especially using age-adjusted D-dimer cut-off levels (39).
Moreover, it is worth to mention that in our study population there were 96 (25.9%) patients with malignancy while in the Geneva score study (40) only 13% [138] of patients in their cohort had malignancy. Karamat et al. assessed the utility of D-dimer test in predicting PE in 104 cancer patients (41) and we had similar sensitivity (94.6% vs. 95.5%), lower specificity (6.8% vs. 28.2%), as well as the PPV (38.9 vs. 42.8) and the NPV (66.7% vs. 91.6%) was lower.
It should also be underlined that in our study a negative D-dimer test does not exclude the presence of acute PE 100% of the time, because 12.7% of the patients with normal plasma D-dimer levels had confirmed PE. Furthermore, among all patients with confirmed PE in our study, 7 of 134 (5.2%) had normal D-dimer concentrations. An earlier study reported an even higher percentage of PE patients with normal D-dimer values—26% (42). Whereas other studies, in agreement with our results, reported that 3.4% of 382 patients (43), 3.6% of 55 patients (44), and 4% of 725 patients (45) with PE had normal plasma D-dimer values, which is compatible with our results. Another study has shown that 2 of 5 (40%) patients with normal with plasma D-dimer levels had PE or DVT (46). These results are comparable with our results, where 3 of 7 (42.8%) patients with plasma D-dimer levels <500 ng/mL had acute PE. Another study also encourages physicians to search for acute PE in patients with normal D-dimer values, when symptoms can’t be explained otherwise (can’t be connected with another disease), in the presence of thromboembolism risk factors and when duration of symptoms is unnaturally long (45). We also considered what could explain negative D-dimer results on those patients. Most often false-negative results are caused by anticoagulation therapy (47)—all patients in our study were hospitalized so they could use antithrombotic prophylaxis and 3 of them were already treated because of DVT or stroke before PE diagnosis.
The 2014 European Society of Cardiology (ESC) guidelines on the diagnosis and management of acute PE state that a normal plasma D-dimer level renders acute PE or DVT unlikely but also that the quantitative latex-derived assays (used in our study) and a whole-blood agglutination assay have a diagnostic sensitivity <95% and are thus often referred to as moderately sensitive. In outcome studies, those assays proved to be safe in ruling out PE in PE—unlikely patients as well as in patients with a low clinical probability. Their safety in ruling out PE has not been established in the intermediate clinical probability category (36). Our study in combination with previously cited studies (42,43,45,46) confirm results the guidelines, however we suggest, that clinicians should also include information about patients with normal plasma D-dimer level, especially if had previous PE or DVT, duration of symptoms is unnaturally long or when symptoms can’t be explained otherwise.
Our study was based on the retrospective analysis of the medical notes of patients in a hospital database, which prevented us from checking reasons for ordering the plasma D-dimer test and the time frame from symptoms to CTPA. We also did not include cases in which PE was diagnosed without previous D-dimer tests and patients who were diagnosed with acute PE without using CTPA, because contraindicated. However, these cases were few, and it is unlikely that they would have changed the results of our study. Unfortunately, because of the retrospective design of our study we did not have the possibility to use the Geneva score or modified Wells criteria and not always the score of these scales was recorded in the medical notes of our patients, despite the 2014 ESC Guidelines on the diagnosis and management of acute PE recommend to assess the clinical probability of acute PE diagnosis (36). The value of this clinical evaluation has been confirmed in few clinical studies for example the prospective investigation on PE diagnosis (PIOPED) (48). The small number of patients included in our study does not allow to draw clear conclusions about utility risk factors like cancer, pregnancy or post-surgery to obtain different plasma D-dimer cut-off values for those patients and prospective studies in this area are needed.
Conclusions
In general population of patients with plasma D-dimer levels exceeding at least four times (in our study 2,152 ng/mL) its normal value, CTPA should be considered predictive even for patients with a low clinical probability of acute PE. However, the small number of patients included in our study does not allow us to draw clear conclusions about patients with cancer, pregnancy or post-surgery that are also often associated with increased plasma D-dimer levels. Also, the age >65 years and a history of neoplasm, should always be considered in suspecting acute PE, because may significantly increase the plasma D-dimer cut-off level associated with an increased likelihood of acute PE.
Acknowledgements
Funding: This work is supported by Medical University of Silesia (grant number KNW-1-052/N/7/K).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The research was conducted according to the principles of the Declaration of Helsinki. And in Poland (according to the polish law) studies which are not experimental—including retrospective analyses or observational studies—do not require ethical committee approval.
References
- Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol 2012;25:235-42. [Crossref] [PubMed]
- Bronić A. Thromboembolic diseases as biological and clinical syndrome—role of the mediterranean league against thromboembolic diseases. Biochem Med 2010;20:9-12. [Crossref]
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler. Thromb. Vasc. Biol 2008;28:370-2. [Crossref] [PubMed]
- Gupta RT, Kakarla RK, Kirshenbaum KJ, et al. D-Dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol 2009;193:425-30. [Crossref] [PubMed]
- Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol 2009;29:298-310. [Crossref] [PubMed]
- Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 2013;18:129-38. [PubMed]
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:948-54. [Crossref] [PubMed]
- Liu CP, Li XM, Chen HW, et al. Depression, anxiety and influencing factors in patients with acute pulmonary embolism. Chin Med J (Engl) 2011;124:2438-42. [PubMed]
- Conget F, Otero R, Jimenez D, et al. Short-term clinical outcome after acute symptomatic pulmonary embolism. Thromb Haemost 2008;100:937-42. [Crossref] [PubMed]
- den Exter PL, van der Hulle T, Lankeit M, et al. Long-term clinical course of acute pulmonary embolism. Blood Rev 2013;27:185-92. [Crossref] [PubMed]
- Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe—the number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756-64. [Crossref] [PubMed]
- Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3145-6. [PubMed]
- Green SM, Yealy DM. Right-Sizing testing for pulmonary embolism: recognizing the risks of detecting any clot. Ann Emerg Med 2012;59:524-6. [Crossref] [PubMed]
- Gaffney PJ, Creighton LJ, Callus M, et al. Monoclonal antibodies to crosslinked fibrin degradation products (XLFDP). 2. evaluation in a variety of clinical conditions. Br J Haematol 1988;68:91-6. [Crossref] [PubMed]
- Bounameaux H, Slosman D, Demoerloose P, et al. Diagnostic value of plasma d-dimer in suspected pulmonary embolism. Lancet 1988;2:628-9. [Crossref] [PubMed]
- Plasma d-dimer and pulmonary embolism. Lancet 1989;1:791-2. [PubMed]
- Bounameaux H, Cirafici P, Demoerloose P, et al. Measurement of d-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet 1991;337:196-200. [Crossref] [PubMed]
- Ginsberg JS, Wells PS, Brilledwards P, et al. Application of a novel and rapid whole blood assay for didimer in patients with clinically suspected pulmonary embolism. Thromb Haemost 1995;73:35-8. [Crossref] [PubMed]
- Perrier A, Bounameaux H, Morabia A, et al. Diagnosis of pulmonary embolism by a decision analysis-based strategy including clinical probability, D-dimer levels, and ultrasonography: a management study. Arch Intern Med 1996;156:531-6. [Crossref] [PubMed]
- Perrier A, Desmarais S, Goehring C, et al. D-dimer testing for suspected pulmonary embolism in outpatients. Am J Respir Crit Care Med 1997;156:492-6. [Crossref] [PubMed]
- Ginsberg JS, Wells PS, Kearon C, et al. Sensitivity and specificity of a rapid whole-blood assay for D-dimer in the diagnosis of pulmonary embolism. Ann Intern Med 1998;129:1006. [Crossref] [PubMed]
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000;83:416-20. [Crossref] [PubMed]
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001;135:98-107. [Crossref] [PubMed]
- Kruip MJ, Slob MJ, Schijen JH, et al. Use of a clinical decision rule in combination with D-dimer concentration in diagnostic workup of patients with suspected pulmonary embolism—a prospective management study. Arch Intern Med 2002;162:1631-5. [Crossref] [PubMed]
- Ten Wolde M, Hagen PJ, Macgillavry MR, et al. Non-invasive diagnostic work-up of patients with clinically suspected pulmonary embolism; results of a management study. J Thromb Haemost 2004;2:1110-7. [Crossref] [PubMed]
- van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006;295:172-9. [Crossref] [PubMed]
- Becattini C, Lignani A, Masotti L, et al. D-Dimer for risk stratification in patients with acute pulmonary embolism. J Thromb Thrombolysis 2012;33:48-57. [Crossref] [PubMed]
- Ghanima W, Abdelnoor M, Holmen LO, et al. D-dimer level is associated with the extent of pulmonary embolism. Thromb Res 2007;120:281-8. [Crossref] [PubMed]
- Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149: 481-90, W94.
- Ji Y, Sun B, Juggessur-Mungur KS, et al. Correlation of D-dimer level with the radiological severity indexes of pulmonary embolism on computed tomography pulmonary angiography. Chin Med J (Engl) 2014;127:2025-9. [PubMed]
- Shah K, Quaas J, Rolston D, et al. Magnitude of D-dimer matters for diagnosing pulmonary embolus. Am J Emerg Med 2013;31:942-5. [Crossref] [PubMed]
- Tick LW, Nijkeuter M, Kramer MHH, et al. High D-dimer levels increase the likelihood of pulmonary embolism. J Intern Med 2008;264:195-200. [Crossref] [PubMed]
- Legnani C, Cini M, Scarvelis D, et al. Multicenter evaluation of a new quantitative highly sensitive D-dimer assay, the Hemosil D-dimer HS 500, in patients with clinically suspected venous thromboembolism. Thromb Res 2010;125:398-401. [Crossref] [PubMed]
- Arnautović-Torlak V, Pojskić B, Zutic H, et al. Values of D-dimer test in the diagnostics of pulmonary embolism. Med Glas (Zenica) 2014;11:258-63. [PubMed]
- Di Nisio M, Squizzato A, Rutjes AW, et al. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost 2007;5:296-304. [Crossref] [PubMed]
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the european society of cardiology (ESC). Eur Heart J 2014;35:3033-69, 3069a-k.
- Raimondi P, Bongard O, Demoerloose P, et al. D-dimer plasma concentration in various clinical conditions—implication for the use of this test in diagnostic approach of venous thromboembolism. Thromb Res 1993;69:125-30. [Crossref] [PubMed]
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014;311:1117-24. [Crossref] [PubMed]
- Wilts IT, Le Gal G, den Exter PL, et al. PO-29—age-adjusted D-dimer cutoff level increases the number of cancer patients in who pulmonary embolism can be safely excluded without CT-PA imaging: the ADJUST-PE cancer substudy. Thromb Res 2016;140 Suppl 1:S187. [Crossref] [PubMed]
- Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006;144:165-71. [Crossref] [PubMed]
- Karamat A, Awan S, Hussain MG, et al. Usefulness of clinical prediction rules, D-dimer, and arterial blood gas analysis to predict pulmonary embolism in cancer patients. Oman Med J 2017;32:148-53. [Crossref] [PubMed]
- Kutinsky I, Blakley S, Roche V. Normal D-dimer levels in patients with pulmonary embolism. Arch Intern Med 1999;159:1569-72. [Crossref] [PubMed]
- Gibson NS, Sohne M, Gerdes VEA, et al. The importance of clinical probability assessment in interpreting a normal d-dimer in patients with suspected pulmonary embolism. Chest 2008;134:789-93. [Crossref] [PubMed]
- Dunn KL, Wolf JP, Dorfman DM, et al. Normal D-dimer levels in emergency department patients suspected of acute pulmonary embolism. J Am Coll Cardiol 2002;40:1475-8. [Crossref] [PubMed]
- Guo Z, Ma Q, Zheng Y, et al. Normal blood D-dimer concentrations: do they exclude pulmonary embolism? Chin Med J (Engl) 2014;127:18-22. [PubMed]
- Parent F, Maitre S, Meyer G, et al. Diagnostic value of D-dimer in patients with suspected pulmonary embolism: results from a multicentre outcome study. Thromb Res 2007;120:195-200. [Crossref] [PubMed]
- Couturaud F, Kearon C, Bates SM, et al. Decrease in sensitivity of D-dimer for acute venous thromboembolism after starting anticoagulant therapy. Blood Coagul Fibrinolysis 2002;13:241-6. [Crossref] [PubMed]
- Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med 2006;119:1048-55. [Crossref] [PubMed]