Unravelling complexities of the subsolid pulmonary nodule—detection, characterization, natural history, monitoring and (future) patient management
Lung cancer is a major cause of death worldwide with an estimated 1.8 million new diagnoses and 1.6 million deaths annually (1). The five-year survival rate is poor as symptoms usually occur in an advanced stage of cancer where treatment options are limited and no curative therapies are available. Detecting suspicious lesions at an early stage is therefore thought to improve overall lung cancer survival and for this reason several lung cancer screening trials with chest computed tomography have been employed. In addition, the use of chest computed tomography (CT) in clinical care has sharply risen in the past decade. This has taught us important lessons on pulmonary lesions that look like cancer and even have malignant cells in histology, but do not behave malignant. This observation is quite similar to indolent lesions in other organs (2).
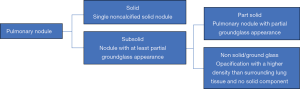
Not every pulmonary nodule is the same, some are solid, some are nonsolid (ground glass) and some are both solid and nonsolid (part-solid). Nonsolid and part-solid nodules are together termed subsolid pulmonary nodules (SSN) (Figure 1). Most literature focused on the best-known lesion present on CT being a solid nodule, but in the last years, knowledge on the SSN has increased. In 2002, Henschke et al. (3) discovered that the histological cancer rate was significantly higher in SSNs as compared to solid nodules, but that the growth rate and metastatic potential was much lower and the prognosis much better for many of these lesions. Since then, numerous publications supported this.
Recently, in Heart Lung Circulation, Tang et al. showed results of a retrospective study with the aim to assess the natural course of subsolid nodules in terms of nodule growth (4). From 2002 to 2016 they included 128 patients with persistent subsolid nodules of 3 cm or smaller. The mean (standard deviation) follow-up period was 3.57±2.93 years. In this study the primary endpoint was cumulative change determined by CT and growth as defined by several thresholds (true diameter growth of ≥2 mm, substantial diameter growth of ≥5 mm, and tumor stage shift). Of the 128 subsolid nodules, 93 (72.7%) were pure ground glass nodules and 35 (27.3%) were part-solid. The 5-year progression rate (based on true growth) of the part-solid group was 67.3% whereas subjects with pure ground glass nodules showed a progression rate of 35.5%. Median progression time for part-solid nodules was 3 years and for ground glass nodules 7 years. For T-stage shift, ground glass nodules took a median follow-up of 12 years, while part-solid nodules took a median follow-up of 9 years. Nodule growth was associated with age, the proportion of the solid component, and follow-up duration. Here we discuss several elements with additional literature that can aid in unravelling this challenging entity.
Detection and characterization of SSN
SSNs can be divided into nonsolid (ground glass) and part-solid nodules (Figure 1), although some investigators even defined an in-between category (5). Some of these SSNs seem to behave non-malignant (do not metastasize for many years) but histologically often malignant cells are detected, especially in the part-solid nodules. The prevalence of invasive adenocarcinomas in part-solid nodules is associated with the absolute size and volume percentage of the solid component and therefore in guidelines nodule management differs based on the size of the solid component (6).
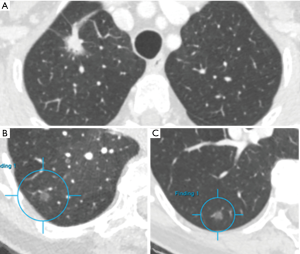
For human observers detection of pulmonary nodules may be challenging, but the major difficulty with SSNs is the characterization in pure ground glass versus part-solid. Van Riel et al. showed only moderate agreement in nodule classification even between expert readers (7). Detection of lung nodules is more and more performed automatically using dedicated software that includes computer-aided detection and diameter or volumetric measurements, but characterization is more challenging and less advanced in terms of automation. Even the Lung-RADS category 4X has been proposed that allows the observer to upgrade a nodule if the observer considers the nodule more suspicious based on subjective visual malignant indicators. With this ‘upgrade’ the patient will undergo a more intensive diagnostic workup (8). Malignant indicators such as spiculation or retraction are morphologic criteria that are prone to subjectivity and make the establishment of a classification system difficult. However, as these morphologic features do seem to help differentiate benign from malignant lesions, computer aided analyses that surpass the experience of a human observer should be incited (9). Figure 2A shows an example of a nodule including a large solid core with a small ground glass rim.
Monitoring nodules: mass versus volume versus 2D diameter
Radiologists in most clinical institutions measure the size of SSNs manually, but manual diameters measurements are prone to variability given the spatial resolution of computed tomography and other factors (7). In comparison to solid nodules, SSNs are even more difficult to evaluate because they tend to grow slowly, the border can be less clear defined and also delineating the solid component can be challenging. A recent study among 107 radiologists showed that variation in diameter was higher for the SSN as compared to the solid nodule (10). This study also showed that this variability could lead to different management strategies.
In solid nodules, volume measurements have been shown superior to diameter measurements, but these measurements have not been used regularly for nodules with a ground glass appearance or border. Mass is a parameter that integrates volume and density and can be calculated by multiplying nodule volume and density. This can be of importance as SSN can sometimes shrink in size while there is an increase in number of cells/density. Also, this can overcome some limitations in strict segmentation of the solid core. A disadvantage is that the percentage of solid core may be a relevant parameter in SSNs. As compared to diameter and volume measurements alone, mass measurements have been shown to enable early detection of growth of ground glass nodules (6). The previously published modest interobserver agreement in 2D diameter (especially in smaller SSNs) could be overcome by using automated techniques and indeed, semi-automated volumetric measurements of SSNs have shown to be feasible with good interscan agreement in a more recent study (11). This study showed that when using software, an increase in mass of 30% could be regarded as significant growth. Nodule management guidelines are based on nodule volume doubling time and recently this exponential growth behavior has been confirmed for many nodules in vivo (12).
Natural history and monitoring
Already in 2011 a classification system for adenocarcinomas was published by the International Association for the Study of Lung Cancer (13). This report acknowledged that there is a radiologic spectrum of adenocarcinomas according to histologic subtypes. Knowledge of this spectrum may enhance the comprehension of the natural history of subsolid nodules.
SSNs have been proven to be often precursors of a subgroup of adenocarcinomas which represent 40% of all lung cancers and are therefore of clinical relevance. The spectrum of adenocarcinoma starts with adenomatous atypical hyperplasia (AAH) usually presenting as a faint small pure ground glass nodule on CT and is usually smaller than 5 mm. Larger pure ground glass lesions are thought to represent pre-invasive lesions. A new or growing solid component correlates with the degree of invasiveness (14). Adenocarcinoma in situ (AIS) mostly presents as a ground glass nodule as well, but they can also have a part-solid appearance. An exception is the mucinous AIS, which can be entirely solid on CT. By definition an AIS is smaller than 3 cm. Minimal invasive adenocarcinoma (MIA) presents often as a part-solid nodule in which the ground glass part is mostly larger than the solid part. Invasive adenocarcinoma generally presents as a solid nodule, but may also have a ground glass part, which is usually smaller than the solid part. A special form of invasive adenocarcinoma is the mucinous adenocarcinoma, usually presenting on CT as a consolidation surrounded with ground glass attenuation, often misinterpreted as pneumonia.
Several studies have analyzed the natural course of subsolid nodules and reported the relative indolent clinical course of most of these nodules. Based on these results, the 2017 Fleischner guidelines recommended longer follow-up periods for subsolid nodules (15). The results of Tang et al. support these new guidelines as they showed that part-solid nodules usually grow within 3 years and some subjects showed a shift in tumor stage within 2 years (4). They concluded that a CT follow-up time for at least 3 years is necessitated and surgical resection or biopsy is indicated when lesions show interval growth. The growth pattern of SSNs is usually slow and predictable, therefore management with close follow-up seems to be a safe option to avoid overdiagnosis. These considerations should also take into account life expectancy and co-morbidities (16).
Many SSNs are transient and morphologic features could be of help in discriminating between transient and persistent subsolid nodules. However, SSNs do not have well-established morphologic features predictive of malignancy or persistence and not all persistent subsolid nodules develop into a malignancy. Therefore, several years of follow-up is needed. As limited studies focused on long term outcome, a gap persists in the knowledge on long-term progression of subsolid nodules.
For ground glass nodules the management is even less clear-cut. Ground glass nodules seem to occur more frequent in Asian women and non-smokers, suggesting a different etiology as opposed to non-small cell lung cancer (NSCLC). Indeed, ground glass nodules, in accordance with part-solid nodules, have frequently shown to be part of the adenocarcinoma spectrum. Regarding nodule growth, a larger nodule size (>10-mm) and a history of lung cancer have been found to be significant predictors of growth in nonsolid ground glass nodules (17). Invasive adenocarcinomas were diagnosed only among the part-solid nodules and not in ground glass nodules in a large study of Kakinuma et al. (5) including 1226 lung nodules. The Fleischner guidelines recommend an initial follow-up CT-scan for ground glass nodules at 6 months followed by a CT-scan at 3 and 5 years. Tang et al. considered a mandatory follow-up period of 7–9 years with a 12–24 months interval. Nonetheless, malignant transformation of subsolid nodules is only present in less than 1% of all patients with pure ground glass nodules and therefore this ‘conservative approach’ could involve a large number of unnecessary follow-up CTs. An example of a ground glass nodule barely changing in size and characteristics over 15 years is shown in Figure 2B,C.
Further steps beyond persistence and growth rate
Given the low metastatic potential, slow growth rate and occurrence in sometimes young patients, SSNs demand special thoughts on patient management. Death from metastatic SSNs, excessive numbers of scans and overtreatment should be prevented as much as possible. In this sense the paper by Tang et al. helped as it looked at T-stage shift. Further steps that are even more important are N-stage and M-stage shift. Lymph node metastases are important in non-small-cell lung cancer, but lymph node involvement in subsolid nodules was very uncommon as presented in a review including 20 (mostly Asian) studies (18). In addition, no data has been published yet on the presence of distant metastases for this entity. Therefore, especially in patients with substantial comorbidities and reduced life expectancy, a wait-and-see policy may be extended beyond current recommendations. On the other hand, in patients in their thirties or forties, what would be the point of monitoring lesions for decades that will slowly grow and that may eventually evolve in invasive adenocarcinoma?
A special challenge that has not been fully resolved as well is the co-occurrence of many SSNs in a single patient. This may suggest that a lung is diffusely vulnerable for developing lung cancer. It also seems that lung cancers more often develop in patients with SSNs, at distance from a previous SSN. This topic requires further studies and if so, patients may require (lifelong) monitoring even after resection of SSNs.
More personalized risk prediction models could help in guiding the process of nodule management, but studies thus far were based on a relatively small population. A larger set was published by McWilliams et al. (19) and included variables such as age, sex, family history of lung cancer, emphysema, nodule size, lobe location, nodule count and spiculation. Yet, these models do not help separating SSNs with and without metastatic potential. Survival rates have shown to be very high for patients with lung cancer manifesting as part-solid nodules, especially when the solid part has found to be small. For the latter, disease free survival and overall survival rates have shown to be above 97% (14).
Considering all together, the data obtained shows that selecting people at high risk of developing metastatic lung cancer from subsolid nodules is difficult, but this is key to a more effective screening and personalized management. Many risk models include age, smoking exposure or asbestos exposure, but characteristics of patients with subsolid nodules are different from patients with solid nodules with relatively young non-smoking women of Asian origin. Studies that include ethnical and genetic aspects, as well as environmental risk factors (e.g., cooking fumes) will develop models that can select people at high risk of developing malignant subsolid nodules.
Because the inter-observer variability is substantial for characterizing SSNs and given the fact the nodules concerned may be more complex than dichotomous grouping ground glass versus a part-solid appearance, automatic characterization and quantification may be of use. Automatically extracting features like nodules size, texture and intensity, and deep learning features beyond the human eye may prove to be useful in predicting malignant potential. In the future, artificial intelligence and deep learning may play an increasing role in managing SSN (20). An advantage of deep learning is that no preset classification of nodules is used and the algorithm decides which feature of the nodule is useful for the prediction model. This can further shape our conception of subsolid nodules as this entity represents a spectrum of nodules ranging from pre-invasive to invasive adenocarcinomas to metastatic cancers. A challenge will be that histological invasion may not be the best endpoint in model development, but waiting for N and M metastasis to develop is not ethical. The natural clinical course of SSNs has shown to be relatively indolent and the malignant/metastatic potential is low. Hence, a conservative treatment approach may be suitable with follow-up guidelines that pursue beyond smoking status, interval growth and tumor T-stage shift. Multicenter studies and long-term follow-up will be key to set a next step in optimizing SSN management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Kale MS, Korenstein D. Overdiagnosis in primary care: framing the problem and finding solutions. BMJ 2018;362:k2820. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-57. [Crossref] [PubMed]
- Tang EK, Chen CS, Wu CC, et al. Natural History of Persistent Pulmonary Subsolid Nodules: Long-Term Observation of Different Interval Growth. Heart Lung Circ 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- de Hoop B, Gietema H, van der Vorst S, et al. Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology 2010;255:199-206. [Crossref] [PubMed]
- van Riel SJ, Sánchez CI, Bankier AA, et al. Observer variability for classification of pulmonary nodules on low-dose CT images and its effect on nodule management. Radiology 2015;277:863-71. [Crossref] [PubMed]
- Chung K, Jacobs C, Scholten ET, et al. Lung-RADS category 4X: Does it improve prediction of malignancy in subsolid nodules? Radiology 2017;284:264-71. [Crossref] [PubMed]
- Charbonnier JP, Chung K, Scholten ET, et al. Automatic segmentation of the solid core and enclosed vessels in subsolid pulmonary nodules. Sci Rep 2018;8:646. [Crossref] [PubMed]
- Nair A, Bartlett EC, Walsh SLF, et al. Variable radiological lung nodules evaluation leads to divergent management recommendations. Eur Respir J 2018;52. [Crossref] [PubMed]
- Scholten ET, Jacobs C, van Ginneken B, et al. Detection and quantification of the solid component in pulmonary subsolid nodules by semiautomatic segmentation. Eur Radiol 2015;25:488-96. [Crossref] [PubMed]
- Mets OM, Chung K, Zanen P, et al. In vivo growth of 60 non-screening detected lung cancers: a computed tomography study. Eur Respir J 2018.51. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 2014;9:74-82. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner society. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Scholten ET, de Jong PA, de Hoop B, et al. Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules? Eur Respir J 2015;45:765-73. [Crossref] [PubMed]
- Matsuguma H, Mori K, Nakahara R, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest 2013;143:436-43. [Crossref] [PubMed]
- Yip R, Li K, Liu L, et al. Controversies on lung cancers manifesting as part-solid nodules. Eur Radiol 2018;28:747-59. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Ciompi F, Chung K, van Riel S, et al. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci Rep 2017;7:46479. [Crossref] [PubMed]