Aorto-atrial fistula formation and therapy
Introduction
An aorta-atrial fistula (AAF) is a rare but complex pathological condition that can either be congenital or acquired. Usually AAF is secondary to another pathological condition. An AAF is characterised by the presence of the abnormal intra cardiac flow between the aorta and the left or right atrium. The underlying disease needs to be understood thoroughly to enable planning the optimal therapeutic strategy. Here we present a comprehensive overview of the clinical characteristics, subtypes of AAF, the role of the imaging modalities as well as different therapeutic options to treat AAF. It is hoped that this overview will help clinicians in identifying AAF as well as providing optimal treatment.
Clinical presentation
AAF can affect patients from a broad age range, and has been described in patients ranging from 5 days old to 85 years old (1,2). The clinical presentation of AAF encompasses a wide range of signs and symptoms of heart failure including dyspnoea, chest pain, palpitations, fatigue, weakness coughing or oedema.
As the AAF redirects oxygen rich blood from the aorta through the fistula back into one of the cardiac chambers, the mechanism of heart failure is thought to be due to volume overload and cardiac remodelling. Furthermore, and most likely related to the shunt volume, the AAF can also result into distension of the atrial wall, with or without subsequent occurrence of atrial fibrillation. If blood is redirected directly into the ventricles, extra volume can cause increased ventricular strain and lead to cellular remodelling. Continuous right-sided volume overload would eventually lead to an increase in pulmonary pressure, pulmonary oedema and ultimately the development of right ventricular failure. Physical examination may reveal a continuous murmur at the right upper sternal border. A murmur is, however, a non-specific sign and is not indicative of the severity of the AAF. Despite this, when a new continuous cardiac murmur is detected in patients with a history of cardiac surgery, an occurrence of AAF should be considered. This is especially true when other signs or symptoms of heart failure are present. Furthermore, left-sided volume overload can cause eccentric left ventricular hypertrophy and mitral annular dilation with secondary mitral insufficiency. This will result in a worsening of heart failure symptoms and may alter treatment options.
Types of AAF
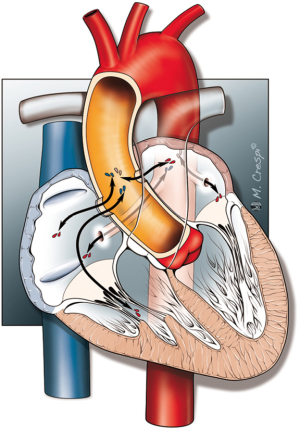
There are a number of manners in which AAF can evolve, and the anatomy of these different configurations are shown in Figure 1.
Congenital AAF
Reports of congenital AAF are less common compared to acquired AAF. Congenital AAF can be subdivided into two major sub-groups, congenital aorto-atrial tunnels and coronary cameral fistulas. Aorto-atrial tunnels are congenital anomalies originating above the sinotubular ridge, caused by an inherited weakness in the aortic wall, leading to the formation of a tunnel into one of the atria (3). Coronary cameral fistulas occur when one of the coronary arteries forms a shunt directly into the atria (3-5). Another very rare anomaly is the idiopathic aortic root (sinus of Valsalva) atrial fistula which was recently described by Campisi et al. (6). Clinical presentation of congenital AAF can range from asymptomatic to severe heart failure. Early correction of these anomalies is therefore recommended due to the fact that the symptoms are likely to worsen over time, with a subsequent higher surgical risk when patients become symptomatic (3,7-9).
Acquired AAF
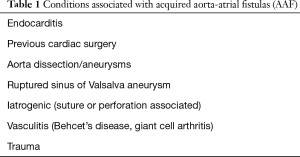
Acquired AAF is associated with a wide range of conditions as shown in Table 1. Endocarditis is one of the most important contributory causes of AAF. The inflammatory processes involved in this disease, together with bacterial destruction of tissue, can weaken the integrity of the aortic and atrial walls and hence increase vulnerability to fistula formation. The incidence of AAF in the setting of endocarditis ranges from 1.6% in patients with native valves up to 5.8% in patients with a prosthetic valve (10). Often a paravalvular abscess coincides with an endocarditis infected aortic root. This can be detected on transesophageal echocardiography (TEE), in the mid-esophageal long axis view (Figure 2). These abscesses can destroy cardiac tissue and protrude into a cardiac cavity. In the majority of infective endocarditis cases, the fistulous communication is between the aorta and the adjacent left atrium (11,12).
Full table

Certain common cardiac surgical procedures can also be associated with AAF formation without endocarditis (Table 2). In cases where infection is not present, fistulas are generally more common between the aorta and the right atrium than aorto-left atrial fistulas. Fistula formation between the left atrium and aorta is very rare due to the anatomical position of these structures. The occurrence of AAF in aortic surgery is much more common compared to coronary artery bypass grafting (CABG) surgery. It is thought that suture-related causes, as well as the location of the suture area, are detrimental factors related to the risk of AAF formation. In patients without a history of cardiac surgery or infection, ruptured sinus valsalva aneurysm, aortic dissections or aneurysms are the major cause of AAF formation.

Full table
Special consideration should also go to patients who have undergone percutaneous closure of atrial septal defects using an occluding device. Erosion through the atrial wall due to the oversizing of the occlude device or a deficient aortic rim can result in fistula formation. It has previously been assumed that motion of the cardiac wall in conjunction with the closing tension of the device may cause the edge of the disk to erode into the aortic wall (13). Therefore, long-term follow-up of these patients is essential, especially when large devices have been implanted. It has also been recommended to avoid the use of closure devices in patients with an aortic rim of less than 5 mm (14).
Systemic diseases associated with vasculitis such as Behcet’s disease or Kawasaki have a higher overall incidence of developing vascular fistula (15,16). For example, we identified two cases of AAF in patients with vasculitis, one patient with Behcet’s disease with no other cardiac anomalies and finally a patient with giant cell arteritis, who had undergone a repair of an aortic dissection (17,18).
Although connective tissue diseases (e.g., Marfan) have a higher incidence of developing vascular abnormalities such as aortic aneurysms, to our knowledge, no reports on AAF are currently available in this patient category. Other incidental causes of acquired AAF reported in literature include chest trauma and placements of extra-cardiac stents with subsequent migration of these stents (19-21).
Imaging techniques to diagnose AAF
Echocardiography
Both TEE and trans-thoracic echocardiography (TTE) can be used to evaluate AAF. Colour Doppler TEE often provides superior image quality in cases where fistulas occur from the posterior aspect of the aorta due to the more optimal alignment of the probe. TTE, on the other hand, renders a better orientation of the atria compared to TEE. 3D-TEE might become the future method of choice for the assessment of AAF because 3D imaging allows spatial orientation and high anatomic definition of adjacent structures (11,22,23).
A major benefit of echocardiography is the possibility to examine cardiac defects in the operating room at all stages of surgery (before, during and after). Possible drawbacks include the requirement of high-frequency transducers with high spatial resolution in certain type of patients, limitations in evaluation of vascular structures positioned outside the heart and sometimes TEE probe insertion is contra-indicated.
Cardiac magnetic resonance imaging (CMR) and cardiac catheterization
CMR has proven to be superior in the quantification of cardiac shunt fraction and flow measurements when compared to echocardiography and computed tomography (CT) alone (24). Sensitivity of CMR to detect aortic vascular anomalies is comparable to angiocardiography. However, with regard to pulmonary artery flow, CMR is the only available technique that allows quantitative flow measurements and is considered the gold standard for assessment of pulmonary vein anomalies (24,25). In cases of intra-cardiac shunts, CMR can be used for anatomical detection and calculation of shunt fraction (26,27). Drawbacks of CMR are its inability to be used intraoperatively and the use of gadolinium-based dye as a contrast medium, which has been associated with nephrogenic systemic fibrosis.
Cardiac catheterization can also be used to accurately assess shunt quantification, but flow measurements are less accurate compared to CMR (24). The drawback of cardiac catheterization is mainly its invasive nature, which has its inherent risks and potential problems.
Therapy of AAF
Once an AAF is diagnosed, the underlying disease needs to be understood thoroughly to plan the optimal therapeutic strategy. Due to the low incidence of AAF, no clinical trials have been performed in these patients and treatment strategies are based on expert opinion and consensus amongst the treating physicians. Correction of an AAF is strongly recommended when the AAF is not trivial. Uncorrected AAF may continue to impose a risk of progression to overt heart failure. The repair of an AAF can either be surgical or percutaneous. Surgical closure of an AAF is usually based on patches or ligation of the fistula tract and percutaneous closure can be achieved by using an occluder device, coil embolization or covered stents. In the case of percutaneous closure with an occluder device, an Amplatz Ductal Occluder device is most commonly used (28). In all patients who undergo percutaneous closure with an Amplatzer device follow-up through postop imaging is necessary to detect if there is a residual fistula or pseudoaneurysm present (29). Given that the majority of information regarding AAF is from case reports, it is difficult to accurately gauge the treatments success rates or mortality rates. However, a recent review of these case reports from our own group indicated that the overall treatment success rate for both surgical and percutaneous closure is above 80%. The mortality rate for both surgical and percutaneous closure using the same data set was around 7% (30).
Small asymptomatic aorto-atrial fistulas can sometimes be managed pharmacologically, by reduction of cardiac afterload and the use of diuretics. Conservative management should involve close monitoring of the patient and if clinical conditions deteriorates, active closure of the fistula should be reconsidered. Clinicians should always keep in mind that even asymptomatic and small AAF have a tendency to worsen over time, with subsequent higher surgical risk when patients become symptomatic.
Conclusions
AAF is a relatively rare but very serious condition. Clinicians should consider the possibility of AAF, when a new continuous cardiac murmur occurs, especially in patients with a history of cardiac surgery or with signs of heart failure. Pre-operative localisation and quantification of intra-cardiac shunts can best be studied using CMR, whereas echocardiography is ideal to provide perioperative assessment and guidance for the surgeon. In the absence of clinical trials, clinical approach in treating AAF is based on expert opinion and consensus. Closure of the AAF fistula tract is generally recommended, even if the fistula is asymptomatic, based on observations that AAF have a tendency to worsen over time. Further studies would be required to define optimal therapeutic strategies, but these are hindered by the rarity of the occurrence of this disorder.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Danilowicz D, Presti S, Colvin S, et al. Congenital fistulous tract between aorta and right atrium presenting as heart failure in a newborn. Pediatr Cardiol 1989;10:93-7. [Crossref] [PubMed]
- Matsuhisa H, Obo H, Nakagiri K, et al. Aorto-right atrial fistula caused by type A aortic dissection. Ann Thorac Surg 2004;78:2173-5. [Crossref] [PubMed]
- Sai Krishna C, Baruah DK, Reddy GV, et al. Aorta-right atrial tunnel. Tex Heart Inst J 2010;37:480-2. [PubMed]
- Nihoyannopoulos P, Sapsford R, Oakley CM. Congenital fistula between the aorta and left atrium. Br Heart J 1987;57:387-90. [Crossref] [PubMed]
- Elwatidy AF, Galal AN, Rhydderch D, et al. Aorto-right atrial fistula. Ann Thorac Surg 2003;76:929-31. [Crossref] [PubMed]
- Campisi S, Cluzel A, Vola M, et al. Idiopathic Aortic Root to Right Atrial Fistula. J Card Surg 2016;31:373-5. [Crossref] [PubMed]
- Türkay C, Gölbaşi I, Belgi A, et al. Aorta-right atrial tunnel. J Thorac Cardiovasc Surg 2003;125:1058-60. [Crossref] [PubMed]
- Gajjar T, Voleti C, Matta R, et al. Aorta-right atrial tunnel: clinical presentation, di-agnostic criteria, and surgical options. J Thorac Cardiovasc Surg 2005;130:1287-92. [Crossref] [PubMed]
- Aggarwal SK, Sai V, Iyer VR. Imaging features of aorto-right atrial tunnel: a report of two cases. Congenit Heart Dis 2007;2:429-32. [Crossref] [PubMed]
- Anguera I, Miro JM, Vilacosta I, et al. Aorto-cavitary fistulous tract formation in infective endocarditis: clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur Heart J 2005;26:288-97. [Crossref] [PubMed]
- Patsouras D, Argyri O, Siminilakis S, et al. Aortic dissection with aorto-left atrial fistula formation soon after aortic valve replacement: A lethal complication diagnosed by transthoracic and transesophageal echocardiography. J Am Soc Echocardiogr 2002;15:1409-11. [Crossref] [PubMed]
- Archer TP, Mabee SW, Baker PB, et al. Aorto-left atrial fistula. A reversible cause of acute refractory heart failure. Chest 1997;111:828-31. [Crossref] [PubMed]
- Mello DM, Fahey J, Kopf GS. Repair of aortic-left atrial fistula following the transcatheter closure of an atrial septal defect. Ann Thorac Surg 2005;80:1495-8. [Crossref] [PubMed]
- Grayburn PA, Schwartz B, Anwar A, et al. Migration of an amplatzer septal occluder device for closure of atrial septal defect into the ascending aorta with formation of an aorta-to-right atrial fistula. Am J Cardiol. 2005;96:1607-9. [Crossref] [PubMed]
- Chung HJ, Goo BC, Lee JH, et al. Behçet’s disease combined with various types of fistula. Yonsei Med J 2005;46:625-8. [Crossref] [PubMed]
- Liang CD, Kuo HC, Yang KD, et al. Coronary artery fistula associated with Kawasaki disease. Am Heart J 2009;157:584-8. [Crossref] [PubMed]
- Haddad F, El-Rassi I, Haddad FG, et al. Aorto-atrial fistula 10 days after dissection repair in giant cell arteritis. Ann Thorac Surg 2008;86:1672-4. [Crossref] [PubMed]
- Melua A, Campbell N, McCluskey D, et al. Aorto-atrial fistula without aneurysm formation in Behçet’s disease. Heart 1998;80:200-1. [Crossref] [PubMed]
- Rubin S, Falcoz PE, Poncet A, et al. Traumatic aorto-right atrial fistula and tricuspid valve rupture. Post-operative cardiac and respiratory support with extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 2006;5:735-7. [Crossref] [PubMed]
- Sehgal M, Brown DB, Picus D. Aortoatrial fistula complicating transjugular intrahepatic portosystemic shunt by protrusion of a stent into the right atrium: radiologic/pathologic correlation. J Vasc Interv Radiol 2002;13:409-12. [Crossref] [PubMed]
- Barrio-López MT, Martín-Trenor A, Mastrobuoni S, et al. Iatrogenic atrial septal defect and aortoatrial fistula in a patient with endovascular prosthesis in the inferior vena cava. Ann Thorac Surg 2012;93:e23-25. [Crossref] [PubMed]
- Bouchez S, Wouters PF, Vandenplas G. Asymptomatic aorto-atrial fistula identified with intraoperative transesophageal echocardiography. J Cardiothorac Vasc Anesth 2012;26:e76-77. [Crossref] [PubMed]
- Patsouras D, Potsis T, Siogas K. Echocardiographic evolution of prosthetic aortic valve endocarditis with aorto-left atrial fistula formation. J Am Soc Echocardiogr 2009;22:210.e5-6. [Crossref] [PubMed]
- Valsangiacomo Buechel ER, Grosse-Wortmann L, Fratz S, et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur Heart J Cardiovasc Imaging 2015;16:281-97. [Crossref] [PubMed]
- Riesenkampff EM, Schmitt B, Schnackenburg B, et al. Partial anomalous pulmonary venous drainage in young pediatric patients: the role of magnetic resonance imaging. Pediatr Cardiol 2009;30:458-64. [Crossref] [PubMed]
- Beerbaum P, Körperich H, Barth P, et al. Noninvasive quantification of left-to-right shunt in pediatric patients: phase-contrast cine magnetic resonance imaging compared with invasive oximetry. Circulation 2001;103:2476-82. [Crossref] [PubMed]
- Körperich H, Gieseke J, Barth P, et al. Flow volume and shunt quantification in pediatric congenital heart disease by real-time magnetic resonance velocity mapping: a validation study. Circulation 2004;109:1987-93. [Crossref] [PubMed]
- Alkhouli M, Almustafa A, Kawsara A, et al. Transcatheter closure of an aortoatrial fistula following a surgical aortic valve replacement. J Card Surg 2017;32:186-9. [Crossref] [PubMed]
- Song J, Ascherman B, Eudailey KW, et al. Long-term failure of Amplatzer plugs in the treatment of aortic pathology. J Card Surg 2017;32:426-9. [Crossref] [PubMed]
- Jainandunsing JS, Linnemann R, Bouma W, et al. Aorto-atrial fistula formation and closure: a systematic review. J Thorac Dis 2019;11:1031-46.