
Outcomes comparison between neoadjuvant chemotherapy and adjuvant chemotherapy in stage IIIA non-small cell lung cancer patients
Introduction
Lung cancer makes up more than one-fourth of the estimated death caused by all kinds of malignant tumors annually, of which, non-small cell lung cancer (NSCLC) is the majority (1). The 5-year relative survival rate of NSCLC goes down along the stage at diagnosis, while the stage IIIA is in the gray zone between early-stage lung cancer and late-stage lung cancer, making it at the edge of the technically resectable disease with a potential metastasis (2). Thus, single-modality therapy, surgery or chemotherapy alone, have proven to be unsatisfactory for improving the non-relapse or long-term survival rates. The traditionally recommended therapeutic strategy for stage pIIIA NSCLC is surgery plus an adjuvant platinum-based chemotherapy alone or combined with radiation, with neoadjuvant chemotherapy (NCT) followed by surgery introduced as an alternate option (3).
Since the early 1990s, the efficacy of NCT in mid-early stage lung cancer has been carefully studied. Two randomized controlled trials (RCTs) each containing 60 stage IIIA NSCLC patients [UICC/AJCC TMN stage published in 1986 (4)] compared cisplatin-based NCT plus surgery with surgery alone, both of which demonstrated an absolute survival benefit of NCT (5,6). Afterward, robust studies of RCT and meta-analysis further verified this conclusion (7-11). Neoadjuvant/adjuvant taxol carboplatin hope (NATCH) was the first RCT to directly compared NCT and postoperative adjuvant chemotherapy (ACT). This three-arm trail also contained a surgery-alone arm, and the patients ranged from stages I (tumor size >2 cm), II, and T3N1 (12). Since then, a number of studies have been carried out seeking to evaluate the importance of chemotherapy’s timing in NSCLC (13). Although these studies mainly include a wide range of tumor stages that are unspecific to stage IIIA, no definitive conclusion has been reached.
For the above reasons, we initiated this study to evaluate the influence of NCT followed by radical surgery on short-term and long-term clinical outcomes of stage IIIA NSCLC patients in comparison with surgery plus ACT. Also, we tried to find a specific subgroup of studied patients who might prognostically benefit from NCT or better respond to NCT.
Methods
Cases of the NCT group
We initially reviewed 78 clinical stage IIIA NSCLC patients (the TNM classification of the UICC 8th ed.) who received at least one cycle of platinum-based preoperative chemotherapy (and no less than four cycles in total) followed by lung resection and systematic lymph node dissection between 2007 and 2016 in our institution. The clinical staging methods included enhanced chest computed tomography scanning (CT), brain magnetic resonance imaging (MRI), whole-body bone scanning (SPECT), and cervical and abdominal ultrasonography. The assessment of chest lymph nodes was only performed by enhanced chest CT scanning. Patients who had a history of other malignancy or were defined with R1 or R2 residual lesions were excluded. Finally, there were 68 patients retrospectively enrolled in the NCT group (Figure 1A).

Cases of the ACT group
To make a comparison, we also reviewed cases that received at least four cycles of platinum-based ACT after lung resection and systematic lymph node dissection with stage pIIIA NSCLC (the TNM classification of the UICC 8th ed.) during the same period as the NCT group. After the exclusion of patients with a history of other malignancies, defined with R1 or R2 residual lesions, who received preoperative targeted therapy or targeted adjuvant therapy, or who did not undergo postoperative chemotherapy, a total of 535 patients remained (Figure 1B).
Patients assessment and follow-up
The responses to NCT were assessed according to the response evaluation criteria in solid tumors (RECIST). We defined the start point of follow-up as the first-day patients who received anti-tumor treatments (NCT for NCT group, or surgery for ACT group). Patients were regularly followed up with once per 3 months during the first two years after surgery and then once per year afterward. Physical examination, history acquirement, chest CT scan, cervical and abdominal ultrasonography were required for return visit each time, while brain MRI and SPECT were performed at least once a year. PET-CT was not compulsory. The follow-up information was obtained through a digital clinic system and telephone surveys. Our study received the approval from the Fudan University Shanghai Cancer Center Institutional Review Board (No. 090977-1), and all of the patients who underwent surgery, signed informed consent.
Statistical methods
We carried out t-test or nonparametric tests to compare numeric or categorical variables between the two groups, respectively. Propensity score matching (PSM) was made by the nearest matching method. The 1:1 matching ratio and the caliper was set 0.05 to balance the potential selective bias. Survival analysis was made through a log-rank test. Regression analysis of the Cox proportional hazards regression model and binomial logistic regression model (method: enter) were carried out to find a possible association between the clinical outcomes and features. The software we used for data collection and analysis was Excel (Version 14.3.9, Microsoft Office), SPSS (Version 23.0, IBM-SPSS Inc.) and R (Rstudio Version 1.1.442, RStudio, Inc.). The R packages used were Survival, MatchIt, and plyr.
Results
Patients characteristics
There were 68 patients in the NCT group and 535 patients in the ACT group. Different inclusion criteria of the NCT group and ACT group resulted in significant differences in some variables before PSM including gender (P=0.0153), year of surgery (P<0.0001), smoking history (P=0.0222), central or peripheral location (P<0.0001), pathology (P=0.0002), clinical T stage (P=0.0513), clinical N stage (P<0.0001), and approach of surgery (P=0.0172). Patients who received NCT tended to be males, were former or current smokers, had central NSCLC, and were clinically diagnosed as stage N2, when compared to the ACT group (Table 1).
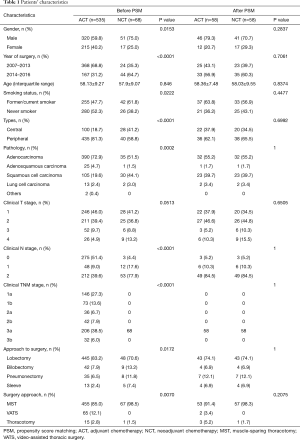
Full table
Surgery duration, hospital stay, and short-term postoperative complications were compared across the two groups after PSM. There was no statistically distinct difference between the ACT and NCT groups (Table 2).
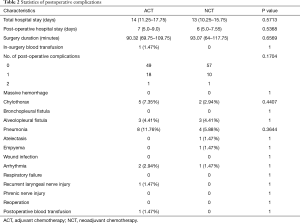
Full table
Survival and recurrence
During the follow-up, there were 358 relapses (34 of 68 in NCT, 50.00%; 324 of 535 in ACT, 60.6%) and 186 deaths (15 of 68 in NCT, 22.06%; 171 of 535 in ACT, 32.0%) reported. The median duration of the follow-up time was 31.98 months. As shown in Figure 2A,B, the relapse-free survival (RFS) and OS before PSM showed there to be no significant difference between the NCT and the ACT groups. After PSM, 10 and 477 cases were omitted in the NCT group and ACT group respectively. After this, both groups comprised 58 patients, and there was no significant difference when baseline clinical characteristics were compared. The difference in RFS and OS between the two groups did not reach statistical significance (Figure 2C,D).

During regression analysis based on the multivariate Cox proportional hazards model, clinical stage T2 was deemed to be an independent predictor of a worse RFS. Squamous cell carcinoma was associated with an improved RFS, and bilobectomy was a worse-OS indicator. Additionally, large cell lung carcinoma and clinical N2 stage were independent predictive factors of a worse RFS and OS. NCT revealed no signs of association with RFS or OS (Table 3).
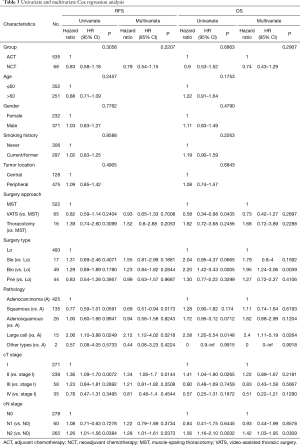
Full table
Subset analysis
Now that grouping of ACT or NCT was revealed to have no significant influence on OS and RFS of overall stage IIIA NSCLC patients, it was important to then clarify if the patients would differ in some subgroups with a particular clinical characteristic or characteristics. To find such a subgroup, we conducted survival analysis on each subset. ACT and NCT of each subgroup was also matched through PSM. For patients in the subgroups of male or female, current/former smoker or never smoker, pathology of adenocarcinoma or squamous cell carcinoma, central or peripheral cancer, clinical T1 or T2, clinical N2, NCT did not represent significant survival benefit over ACT (Table 4).
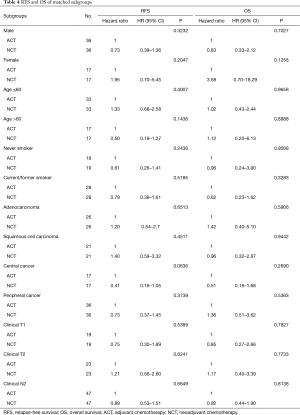
Full table
Response to NCT
We then tested whether or not tumor response to NCT would work as a predictor for a prognosis in the NCT group. The RECIST responses were evaluated through the changes on a CT scan before and after a NCT. Among the 68 patients in the NCT group, there were 34 (50.00%) patients who had partial response (PR), 31 (45.59%) who had stable disease (SD), and 3 (4.41%) who had progressive disease (PD). After the PSM, there were 29 PR, 27 SD, and 2 PD left. We compared the RFS and OS grouped into PR, SD/PD, and ACT. The OS of the PR patients was better than the SD/PD and ACT group (Figure 3). To further investigate predictors for NCT response, we conducted binary logistic regression assessing the association between tumor response and clinical features including gender, age, smoking history, tumor location, pathology, clinical T stage, and clinical N stage; however, none of the features were proven to be a predictive factor (Table 5).

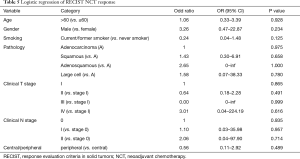
Full table
Discussion
NCT is considered to be a more beneficial choice over ACT in some clinical aspects: it can reduce tumor size, providing a higher possibility of complete tumor resection; it can offer additional time for possible anti-pneumonia treatment, smoke cessation and blood pressure control before surgery; as a preoperative systemic treatment, it can enable the monitoring of chemotherapy response through target lesions, and management of micro-metastasis diseases. The potential risk, meanwhile, is that the planned surgery may be delayed or even canceled because of disease progression during NCT or adverse NCT side effect (14,15).
Previous studies had confirmed the survival advantages of chemotherapy (no matter before or after surgery) over surgery alone in stage IB–IIIA NSCLC (5,7-11,16), while the importance of the timing remained unclarified. Formerly, only a few studies directly compared the clinical outcomes between NCT and ACT. A three-arm RCT, neoadjuvant/adjuvant taxol carboplatin hope (NATCH) demonstrated that the 5-year disease-free survival rate (36.6% in NCT arm, 31.0% in ACT arm) and the 5-year survival rate (41.3% in NCT arm, 36.6% in ACT arm) were similar in the NCT and ACT arms of stage II or T3N1 NSCLC patients (12). A meta-analysis, which abstracted data from 22 trials administrating ACT and 10 trials administrating NCT, also revealed a similar disease-free survival rate (ACT vs. NCT, HR =0.96, 95% CI: 0.77–1.20, P=0.70) and overall survival (OS) rate (ACT vs. NCT, HR =0.99, 95% CI: 0.81–1.21, P=0.71) through indirect comparison meta-analysis (13). The findings from our data, indicate a non-significant survival difference of NCT over ACT, echoed by the above reports. Meanwhile, there were some differences: as opposed to in an earlier stage, our study clarified which study group received more effects of chemotherapy in clinical stage IIIA NSCLC (17); our study was more consistent with more modern NSCLC components, such as more females and more adenocarcinomas (18). In furthering attempt to look for the specific subgroup that might benefit from a failed NCT, more cases should be collected for such a subgroup to be found.
On the other hand, the survival difference between PR and SD/PD according to RECIST response criteria indicated the predictive potential of NCT to evaluate chemotherapy response and long-term survival. However, we could not find a predictor for PR response through logistic regression, because in the retrospective study, the preoperative factors we could gather, especially those considered highly associated with chemosensitivity [e.g., genetic variations (19)], were quite limited. Also, it is hard to tell whether the PR patients can also receive a prolonged survival rate, even if they undergo ACT instead of NCT, as we could not find a subset of ACT patients with the specific predictive factors to compare their survival rates. The only certain conclusion is that if patients have PR response for NCT according to their CT scans, they will have a better prognosis than the SD/PD or the general ACT patients. In contrast, a phase II study published in 2005 revealed a non-significant difference in OS (P=0.25) and DFS (P=0.16) between radiographic responders and non-responders which had been assessed by both CT and PET-CT; the patients were staged from IB to IIIA (20).
Meanwhile, another retrospective study containing 160 NSCLC patients ranged from stage I to stage IV illustrated a significant association between the CT-based RECIST response and OS (P=0.03) (21). Notably, both types of research highlighted a stronger prediction of histopathologic response, suggesting a relative inaccuracy in the tumor-size measurement of a CT scan. Interestingly enough, a study combining two phases II clinical trials demonstrated that RECIST response based on CT, but not PET-CT was prognostic of survival for resectable NSCLC after NCT (22).
Despite similar long-term survival rates, short-term clinical outcomes were illustrated without a statistically distinct difference between the two groups. The results disproved the assumption that NCT allowing sufficient preoperative management would reduce postoperative complications.
This study has some limitations. First of all, patients of the NCT group were clinical stage IIIA NSCLC diagnosed on CT scan, while those in ACT group were pathological stage IIIA NSCLC based on the postoperative diagnosis. Although the clinical stage of the two groups was identical after PSM, the inflammation and fibrosis adjacent to tumors could confound and “up-stage” pretreatment CT diagnosis thus conferring better RECIST response and survival outcomes to NCT patients even if they received anti-inflammatory therapy instead of anti-tumor therapy (21). Also, some of the patients with PD after preoperative chemotherapy may do not undergo subsequent surgery and could end up being excluded from our study. The two above possibilities could skew the results in favor of a better survival outcome of NCT. Also, as retrospective clinical research, our study was restricted to gathering large cases, and so collecting more detailed preoperative clinical information would enable a better-rounded PSM rebalance and more fruitful subgroup analysis. For these reasons, it is necessary to conduct a randomized prospective trial with patients staged through PET-CT, surgical staging of the mediastinum, or EBUS / EUS (23,24).
Nowadays, studies are emerging which utilize treatments other than neoadjuvant therapy. For instance, the Swiss cooperative group, SAKK, showed no additional benefit from adding radiotherapy to NCT followed by surgery (25). Several single-armed studies evaluating the efficacy and toxicity of preoperative erlotinib (26) or PD-1 blockage (27,28) have provided positive results, while most RCTs are still ongoing (e.g., NCT03425643, NCT03456063). With robust evidence testifying the therapeutic effect of targeted therapy and immunotherapy, we think these evolved treatments are more promising for stage IIIA NSCLC.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (Grant number: 81572264, 81601994 and 81572253) and Chinese Minister of Science and Technology (Grant number: 2016YFA0501800, 2017YFA0505500).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Fudan University Shanghai Cancer Center Institutional Review Board (No. 090977-1) and written informed consent was obtained from all patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Van Schil PE, Yogeswaran K, Hendriks JM, et al. Advances in the use of surgery and multimodality treatment for N2 non-small cell lung cancer. Expert Rev Anticancer Ther 2017;17:555-61. [Crossref] [PubMed]
- Jeremic B, Casas F, Dubinsky P, et al. Combined modality therapy in Stage IIIA non-small cell lung cancer: clarity or confusion despite the highest level of evidence? J Radiat Res 2017;58:267-72. [Crossref] [PubMed]
- Mountain CF. A new international staging system for lung cancer. Chest 1986;89:225S-33S. [Crossref] [PubMed]
- Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Pisters KM, Vallieres E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843-9. [Crossref] [PubMed]
- Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 1998;21:1-6. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [Crossref] [PubMed]
- Scagliotti GV. Gemcitabine/cisplatin as induction therapy for stage IIIA N2 non-small-cell lung cancer. Oncology (Williston Park) 2000;14:15-9. [PubMed]
- Chaft JE, Dunphy M, Naidoo J, et al. Adaptive Neoadjuvant Chemotherapy Guided by (18)F-FDG PET in Resectable Non-Small Cell Lung Cancers: The NEOSCAN Trial. J Thorac Oncol 2016;11:537-44. [Crossref] [PubMed]
- Ball D, Mitchell A, Giroux D, et al. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol 2013;8:315-21. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Tan XL, Moyer AM, Fridley BL, et al. Genetic variation predicting cisplatin cytotoxicity associated with overall survival in lung cancer patients receiving platinum-based chemotherapy. Clin Cancer Res 2011;17:5801-11. [Crossref] [PubMed]
- Ramnath N, Sommers E, Robinson L, et al. Phase II study of neoadjuvant chemotherapy with gemcitabine and vinorelbine in resectable non-small cell lung cancer. Chest 2005;128:3467-74. [Crossref] [PubMed]
- William WN Jr, Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222-8. [Crossref] [PubMed]
- Tanvetyanon T, Eikman EA, Sommers E, et al. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J Clin Oncol 2008;26:4610-6. [Crossref] [PubMed]
- Bousema JE, Dijkgraaf MGW, Papen-Botterhuis NE, et al. MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): study protocol of a multicenter randomised controlled trial. BMC Surg 2018;18:27. [Crossref] [PubMed]
- Takeuchi S, Khiewvan B, Fox PS, et al. Impact of initial PET/CT staging in terms of clinical stage, management plan, and prognosis in 592 patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:906-14. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Schaake EE, Kappers I, Codrington HE, et al. Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. J Clin Oncol 2012;30:2731-8. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Yang CJ, McSherry F, Mayne NR, et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:924-9. [Crossref] [PubMed]


