
Fungal infection in lung transplant recipients in perioperative period from one lung transplant center
Introduction
The risk of fungal infection after lung transplantation is high. Fungal infection directly induces the occurrence of relevant complications after surgery and is closely correlated with the mortality of recipients in the perioperative period after lung transplantation (1,2). According to a study (3), the occurrence rate of fungal infection among patients with lung transplantation is approximately at 15–35%, and more than 80% of them are mainly induced by Candida and Aspergillus. Other fungi, such as Trichosporon asahii and Fusarium, are also involved in pathogenic processes. Invasive Candida infection has presented a decreasing occurrence rate among patients, but Aspergillus infection has posed a major risk of poor prognosis among individuals (4). Therefore, fungal distribution after lung transplantation should be investigated to select antibiotics and decrease fungal infection. In this study, we investigated the incidence of fungal infection in 194 patients with lung transplantation in the perioperative period and the fungal distribution among these patients.
Methods
Patients
This study involved a retrospective single-center analysis. Specimens were collected from patients with allogeneic single or bilateral lung transplants in Wuxi People’s Hospital affiliated to Nanjing Medical University from January 2015 to December 2016. A total of 194 individuals received the transplant, and all of the donors came from citizen’s voluntary donations. These donors were declared dead because of cardio-cerebrovascular diseases. The detailed information is shown in Table 1. The recipients and the donors shared the same blood type, presented negative lymphocyte cross matching, and exhibited a panel-reactive antibody <10%. The recipients were provided with regular extracorporeal circulation support and extracorporeal membrane oxygenation for lung transplantation. This study summarized the results of the fungal culture assays for Candida and Aspergillus from the sputum and bronchoalveolar lavage fluid (BALF) of the patients in the perioperative period. Aspergillus infection was diagnosed by detecting galactomannan (GM), which is an antigen of Aspergillus, from the serum of patients. The study was approved by the Research Ethics Board of Wuxi People’s Hospital.
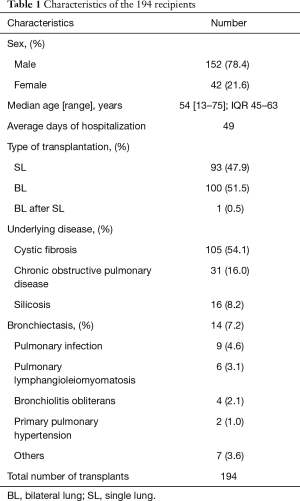
Full table
Definition and categories of fungal infections
In this study, fungal infections were defined and categorized based on fungal classification guidelines according to ISHLT and EORTC criteria (5). Fungal colonization was defined as isolation of fungi in the absence of clinical or radiographic evidence of invasive disease. Pulmonary fungal infection (PFI) based on symptoms, radiology, and histopathology and were categorized into proven, probable, or possible cases. Proven PFI had positive culture, symptoms suggestive of PFI, radiological findings or locally visualized purulent sputum without competing etiology, and positive histopathology or cultured organism from sterile tissue. Probable PFI were defined as those with clinical evidence of infection and positive culture and/or GM antigen assay, and absent or negative histopathology. Possible PFI meet a clinical standard, but there is no microbiological evidence.
Detection of fungal infection
Fungal infection was diagnosed on the basis of the clinical symbols of patients, tomography results, bronchoscopy, microbial detection, pathologic study, and GM detection. The sputum and BALF of patients with potential fungal infection were collected for fungal culture assays. The fungal strains were identified on the basis of morphological characteristics and through microscopic analysis by using standard taxonomic keys. In GM detection, if the result from the sample [sample optical density (OD) value/average OD value of the critical control] was ≥0.5, it was considered positive.
Immunosuppression for the recipients
Cyclosporin A or tacrolimus was orally administered. A large dose of methylprednisolone was injected into the vein, and basiliximab treatment was conducted during the operation and 4 days after the operation to avoid the occurrence of reperfusion of the transplanted lung during operation. After transplantation was completed, tacrolimus or cyclosporin A, mycophenolate mofetil, and prednisone combination treatment was administered to maintain immunosuppression.
Statistical analysis
Descriptive statistics was used to characterize the study population. Chi-square analysis was performed to compare categorical variants based on the statistically analyzed data. Data were examined in SPSS 19.0, and P<0.05 was considered statistically significant.
Results
Fungal distribution before lung transplantation
At least one kind of fungal infection (16.0%) was detected in 31 recipients as observed in the specimens collected from the respiratory system of the 194 recipients. One of the recipients was detected with two kinds of fungi, namely, filamentous fungi and Candida albicans. The detected fungi were mainly Candida (C. albicans, T. glabrata, and C. krusei) and filamentous fungi (Table 2).

Full table
Fungal distribution in the perioperative period after lung transplantation
After lung transplantation, 27 recipients were detected with at least one kind of fungi (13.9%) among the specimens collected from the respiratory system of the 194 recipients. One of the recipients had filamentous fungi and C. albicans. The detected fungi were mainly Candida (C. albicans, T. glabrata, and C. krusei) and filamentous fungi (Table 2). Of the 31 fungal infection-positive cases detected before transplantation, 4 had the same kind of fungi (2.1%). Of these 4 cases, 2 had C. albicans, and 2 had T. glabrata and C. krusei in the perioperative period after transplantation. A total of 54 cases were positive for fungal infection (27.8%) as shown by the summary of the cases before and after transplantation. The most common pathogens causing invasive fungal infections after lung transplantation were Aspergillus spp. and Candida spp. (most commonly C. albicans). Aspergillus infections remain among the most significant opportunistic infections after lung transplantation. The clinical manifestations of filamentous fungal infections were ulcerative tracheobronchitis, invasive pulmonary parenchymal diseases, disseminated multiple organ diseases and/or mycelia. Candida spp. infections often caused fungemia, mediastinitis and pleural space infection.
Detection of Aspergillus antigen (GM)
GM positive was determined in 6 recipients before lung transplantation. The results ranged from 0.503 to 0.833 with an average of 0.603 before transplantation. After transplantation, GM positive was determined in 8 recipients, and the results ranged from 0.504 to 4.311 with an average of 1.423. Of these GM-positive recipients, 4 were detected with filamentous fungal infection before or after transplantation.
Fungal infection in the perioperative period after transplantation
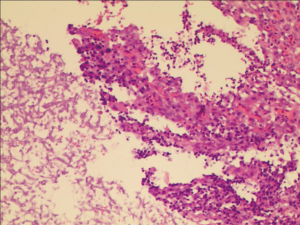
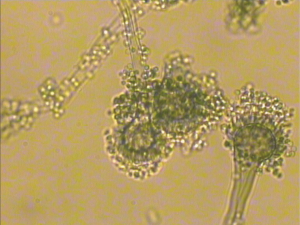
The fungal detection assays of respiratory secretions and the analysis of the clinical symbols revealed that 20 cases of the 194 recipients were detected with fungal infection, including anastomosis infection and bronchial inflammation (10.3%), during the perioperative period after transplantation. Of the 20 cases, 12 and 9 had fungal infection before and after transplantation, respectively, 6 were GM positive, 13 had filamentous fungal infection before or after transplantation, and 9 had confirmed Aspergillus infection (Figures 1,2 and Table 3). There were 3 patients presenting with a post-transplant event by the same class as a pre-transplant event, and they were all colonized with the same species (Aspergillus). Among the 20 patients with post-transplant fungal infection, the 8 patients did not present pre-transplant fungal infection. Although pre-transplant fungal colonization or infection was significantly associated with pre-transplant fungal infection, however, not all patients colonized with fungal would develop subsequent fungal infection. The infection ratio between single- and double-lung transplantation did not significantly differ (χ2=0.0907, P=0.7632). The effects of the basic diseases on the recipients with fungal infection did not also significantly differ (χ2=3.6060, P=0.0576). Among lung transplant recipients with fungal infection, 60% (12/20) of infections were re-infection and 40% (8/20) of infections were de novo infection, respectively. The common pathogen species isolates that we see in lung transplant recipients were Aspergillus and Candida. Compared with the infection ratio between single- and double-lung, the difference was not statistically significant (χ2=0.0907, P=0.7632). Compared with the impact of the basic diseases within the recipients to fungal infection, the difference was not statistically significant (χ2=3.6060, P=0.0576).
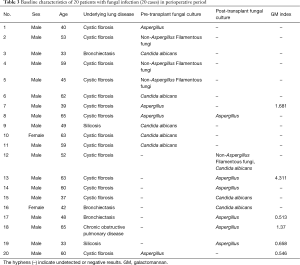
Full table
Discussion
Fungal infection is a common complication among the recipients of lung transplantation. The most common fungi causing infection include Candida and Aspergillus (5). Candida constitutes the majority of the infected fungi (43–80%) in the perioperative period after lung transplantation (3). In our study, 75% of the cases were attributed to Candida. Although Candida is commonly observed in fungal infection, it is usually found in the pharynx oralis. As such, whether the detected Candida is colonized or obtained from external invasion is difficult to determine. Candida infection frequently occurs 1 month after transplantation and is a common cause of invasive fungal infection. Candida infection is usually correlated with ICU duration and Candida colonization before transplantation and surgery. However, Candida rarely causes pulmonary parenchyma infection in the lungs (6).
Aspergillus, which is commonly distributed in the environment, is a common cause of invasive fungal infection. For individuals with a normal immune system, Aspergillus does not cause any invasive Aspergillus-related diseases. However, Aspergillus causes a high risk of disease occurrence for recipients of lung transplantation with a compromised immune system. Luong et al. (7) reported that approximately 40% of lung transplant recipients have Aspergillus colonization in the respiratory system, and 6–16% of them develop invasive Aspergillus-related diseases. Colonization is usually transient, however, it increases the risk of invasive disease. Invasive aspergillosis is considered a major problem in lung transplantation. Patients with lung fibrosis have a high risk of Aspergillus infection. Luong et al. (8) indicated that 70% of patients with pulmonary cystic fibrosis have Aspergillus colonization before lung transplantation, and 22.5% of them develop invasive Aspergillus-related diseases. The average period of the infection is 42 days after transplantation. The occurrence rate of the disease is four times higher than that of the recipients with non-Aspergillus colonization in the respiratory system. The clinical center managing lung transplantation diagnosed a total of 194 recipients before and after lung transplantation. A total of 13 cases (6.7%) with non-repeated filamentous fungal infection were reported. Nine cases of Aspergillus infection, which accounted for 4.6%, were detected. On the basis of the results of a prognosis study on 328 cases of lung transplantation, Hosseini-Moghaddam et al. (4) found that the fungal infection rate within 1 year is 21.6%, and 8.8% of cases are diagnosed as Aspergillus infection. Solé and Salavert (3) retrospectively studied 251 cases of lung transplantation and found that 86 cases (approximately 34%) have Aspergillus infection. Data from different transplantation centers remarkably differ, indicating that the clinical criteria of Aspergillus infection, Aspergillus culture assay, data collection timing, and fungal prophylaxis methods may vary across different centers. In addition, a sensitive biomarker is not established in the diagnosis of invasive Aspergillus infection. Thus, an insufficient frequency of patients for fungal culture assay and Aspergillus antigen detection affects the calculation of the occurrence of Aspergillus infection. The infection rate summarized from our center was comparatively low because of the limited detection on the recently diagnosed patients after transplantation. By contrast, the prophylaxis and treatment of fungi efficiently decreased the occurrence rate of fungal infection. Aspergillus infection in the perioperative period after lung transplantation involves colonization, bronchitis, anastomosis infection, invasive pulmonary aspergillosis (IPA), and disseminated Aspergillus diseases. Although Aspergillus colonization of the respiratory system presents irrelevant symbol at the early stage, the later stage after transplantation usually induces bronchiolitis obliterans syndrome (BOS). Multivariate regression analysis has shown that Aspergillus colonization is an individual risk factor of BOS (9). The death rates between the patients with Aspergillus anastomosis infection in the perioperative period and the patients without an obvious infection symptom were not significantly different. However, a retrospective study showed that Aspergillus colonization decreased the long-term survival rate (4). The occurrence rate of IPA is approximately 5–10% and usually observed in patients with severe immunodeficiency, but the death rate of these patients can reach 80% (6). In the study, one of 20 fungal infection patients died after transplantation was detected with IPA-related during in the perioperative period. Aspergillus fumigatus, the most pathogenic Aspergillus species, produces the most infections, however, Aspergillus flavus, Aspergillus terreus, and Aspergillus niger have been increasingly reported in invasive pulmonary infection. Aspergillus spp. is by far the most common fungal disease caused by fungi, although non-Aspergillus fungi have become an important and increasingly more common pathogens. However, in lung transplant recipients, the scope and overall impact of these emerging fungal pathogens have not been fully determined (3).
The prophylaxis and treatment of fungal infection at the early stage are important because fungal infection after lung transplantation rapidly develops and causes severe outcomes. Anti-fungal drugs are widely used among patients with lung transplantation, including the universal prophylaxis for all recipients after transplantation and preemptive treatment for patients with fungal colonization (4). However, no unified criteria for the prophylaxis and treatment of fungal infection have been presented (10-12). In our center, all patients received intravenous administration of antifungal prophylaxis drugs included by echinococcin or triazole combined with inhaled amphotericin B or liposomal immediately after lung transplant. Because most of the recipients in our center were older and weaker, echinococcins were generally chosen as the initial regimen for systemic drug medication for the comprehensive consideration of the efficacy and safety of drugs. Among the 20 recipients in this group, 17 recipients were given caspofungin or micafungin, however, in the other 3 recipients with Aspergillus colonization or infection before operation, voriconazole was as the first choice. The target prophylaxis strategy should be based on the results of regular bronchoscopy and fungal culture assay. We normally apply fluconazole for C. albicans infection. For Aspergillus colonization, 85% of transplantation centers applied one or two kinds of anti-fungal medicines. The frequently used medicines included oral voriconazole (80.0%). Voriconazole has an anti-fungal capacity for a wide profile of fungal species and low minimum inhibitory concentration (MIC) values. However, its long-term usage is limited by strong liver toxicity and interactions among different immunosuppression medicines. The inhaled amphotericin B treatment has limited toxicity to individuals, can directly target fungal infected tissues, has a high utilization of individuals, and cannot induce drug resistance. For the target prophylaxis strategy, 55% of transplantation centers can proceed from 3 months to 1 year (12). A universal prophylaxis strategy is applied within 3 months after transplantation, and personalized prophylaxis strategies are performed on the basis of different individuals. For patients with Aspergillus colonization before or after lung transplantation, anti-fungal prophylaxis and treatment can last 3 months, thereby efficiently decreasing the occurrence rate of IPA (4,12,13).
For the treatment of IPA, most of the transplantation centers selected 3 to 5 anti-fungal drugs with a course of treatment of more than 1 year. The frequently used drug was voriconazole followed by amphotericin B and echinocandins (e.g., micafungin and caspofungin). Interactions with immunosuppression agents should be considered when recipients take voriconazole (14,15). According to a recent study, some of the cases with Aspergillus fumigatus are resistant to voriconazole and itraconazole (16). The occurrence of voriconazole and itraconazole-resistant molds, or the emergence of secondary resistance to amphotericin B, challenges the choice of antifungal drugs applied as first-line treatment. In our center, the therapeutic strategies included intravenous caspofungin, micafungin, voriconazole, posaconazole, amphotericin B, nebulized amphotericin B or amphotericin B liposome, gargled mycostatin, combined with interventional bronchoscopy. For the treatment of Candida infection after lung transplantation, fluconazole can be applied to treat patients with decreased non-polymorphonuclear leukocytes and patients at a low risk of C. glabrata and C. krusei infections. For patients with severe Candida infection, echinocandins and amphotericin B liposome are the first-line empiric treatment. Empiric treatment should be adjusted on the basis of the results of fungal culture assay and drug sensitivity test. The course of treatment is different based on the infection level and severity of patients (17). Candida treatment normally lasts 2 weeks, and invasive fungal infection persists longer for treatment (18).
In conclusion, our data showed the high risk of fungal infection in the perioperative period after lung transplantation. The main fungal genus was Candida, especially C. albicans. The fungal infection induced by Aspergillus frequently occurred and had a poor prognosis. Bronchoscopy, fungal culture assay, and GM detection should be conducted for patients with a suspected fungal infection in the perioperative period after lung transplantation. GM detection may facilitate the diagnosis of IPA after lung transplantation. But the value of GM is limited, negative results do not exclude invasive fungal infections. The gold standard of diagnosis includes fungal culture and microscopic examination of the infected tissue. It suggested that routine pre-transplant fungal sputum culture, and most importantly, intraoperative BALF culture from the native lung, is useful for identifying higher-risk patients with IPA. It is important for developing efficient targeted preventative strategies to reduce IPA-related the morbidity and mortality. Targeted prophylaxis and treatment should be performed to treat fungal infection on the basis of the results of fungal distribution. However, these processes vary among centers because different transplantation centers have various prophylaxis and treatment strategies against fungal infection. Therefore, relevant strategies from different centers should be summarized to establish the criteria in the selection of anti-fungal drugs and the preparation of dosage and course of treatment.
Acknowledgements
Thanks to Dr. Xianfeng Cheng for linguistic advice.
Funding: This work was supported in part by Medical Science and Research Program of Wuxi (MS201727).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This project was approved by the Research Ethics Board of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (approval No. HS2019011).
References
- Kabbani D, Bhaskaran A, Singer LG, et al. Pentraxin 3 levels in bronchoalveolar lavage fluid of lung transplant recipients with invasive aspergillosis. J Heart Lung Transplant 2017;36:973-9. [Crossref] [PubMed]
- Geltner C, Lass-Flörl C. Invasive pulmonary Aspergillosis in organ transplants--Focus on lung transplants. Respir Investig 2016;54:76-84. [Crossref] [PubMed]
- Solé A, Salavert M. Fungal infections after lung transplantation. Curr Opin Pulm Med 2009;15:243-53. [Crossref] [PubMed]
- Hosseini-Moghaddam SM, Chaparro C, Luong ML, et al. The Effectiveness of Culture-Directed Preemptive Anti-Aspergillus Treatment in Lung Transplant Recipients at One Year After Transplant. Transplantation 2015;99:2387-93. [Crossref] [PubMed]
- Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant 2011;30:361-74. [Crossref] [PubMed]
- Burguete SR, Maselli DJ, Fernandez JF, et al. Lung transplant infection. Respirology 2013;18:22-38. [Crossref] [PubMed]
- Luong ML, Clancy CJ, Vadnerkar A, et al. Comparison of an Aspergillus real-time polymerase chain reaction assay with galactomannan testing of bronchoalvelolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in lung transplant recipients. Clin Infect Dis 2011;52:1218-26. [Crossref] [PubMed]
- Luong ML, Chaparro C, Stephenson A, et al. Pretransplant Aspergillus colonization of cystic fibrosis patients and the incidence of post-lung transplant invasive aspergillosis. Transplantation 2014;97:351-7. [Crossref] [PubMed]
- Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant 2009;9:1903-11. [Crossref] [PubMed]
- Gregson AL. Infectious Triggers of Chronic Lung Allograft Dysfunction. Curr Infect Dis Rep 2016;18:21. [Crossref] [PubMed]
- Peghin M, Monforte V, Martin-Gomez MT, et al. Epidemiology of invasive respiratory disease caused by emerging non-Aspergillus molds in lung transplant recipients. Transpl Infect Dis 2016;18:70-8. [Crossref] [PubMed]
- He SY, Makhzoumi ZH, Singer JP, et al. Practice variation in Aspergillus prophylaxis and treatment among lung transplant centers: a national survey. Transpl Infect Dis 2015;17:14-20. [Crossref] [PubMed]
- Chong PP, Kennedy CC, Hathcock MA, et al. Epidemiology of invasive fungal infections in lung transplant recipients on long-term azole antifungal prophylaxis. Clin Transplant 2015;29:311-8. [Crossref] [PubMed]
- Thakuria L, Packwood K, Firouzi A, et al. A pharmacokinetic analysis of posaconazole oral suspension in the serum and alveolar compartment of lung transplant recipients. Int J Antimicrob Agents 2016;47:69-76. [Crossref] [PubMed]
- Husain S, Sole A, Alexander BD, et al. The 2015 International Society for Heart and Lung Transplantation Guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: Executive summary. J Heart Lung Transplant 2016;35:261-82. [Crossref] [PubMed]
- Shalhoub S, Luong ML, Howard SJ, et al. Rate of cyp51A mutation in Aspergillus fumigatus among lung transplant recipients with targeted prophylaxis. J Antimicrob Chemother 2015;70:1064-7. [PubMed]
- Bedin Denardi L, Hoch Dalla-Lana B, Pantella Kunz de Jesus F, et al. In vitro antifungal susceptibility of clinical and environmental isolates of Aspergillus fumigatus and Aspergillus flavus in Brazil. Braz J Infect Dis 2018;22:30-6. [Crossref] [PubMed]
- Sims KD, Blumberg EA. Common infections in the lung transplant recipient. Clin Chest Med 2011;32:327-41. [Crossref] [PubMed]


