Comparison between monitored anesthesia care and general anesthesia in patients undergoing device closure of atrial septal defect
Introduction
Percutaneous device closure of an atrial septal defect (ASD) is a safe and effective procedure for the complete closure of an ASD in children and adults (1,2). Use of transesophageal echocardiography (TEE) during this procedure is essential to confirm the correct placement of the closure device and to detect complications including early thrombosis, interference with other cardiac structures, and misplacement of the device (3). Although general anesthesia (GA) has usually been used to perform ASD device closures for the protection of airway due to the use of the TEE probe, there have been reports that the procedure can be performed safely under conscious sedation or deep sedation (4,5).
Monitored anesthesia care (MAC) is a type of anesthesia service involving an anesthesiologist who manages the entire process of sedation and analgesia and converts the anesthesia method to GA during the procedure if necessary. During MAC, the depth of sedation can be adjusted from minimal sedation to deep sedation as appropriate. When an ASD device closure was performed under MAC in our center, the deep sedation had been chosen as the sedation depth for the patient’s tolerance to TEE probe insertion and for minimal body movement during the procedure. In this study, we investigated the possibility of using MAC with deep sedation as an alternative to GA in percutaneous device closures of ASDs.
Methods
Study population and data collection
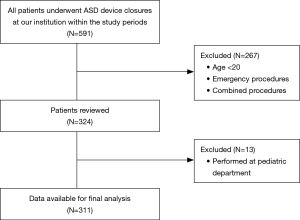
This study was approved by the institutional review board of our center. We retrospectively analyzed the records of patients who underwent percutaneous device closures of ASDs from January 2011 to December 2015 at the Congenital Heart Diseases Center of the Asan Medical Center, Seoul, Korea. Patients who underwent device closures combined with other procedures or operations were excluded; those who underwent emergency procedures or were under 20 years of age were also excluded. Data regarding the baseline characteristics, perioperative variables, and the incidence of complications of the patients were collected from the electronic health records system of our center (Asan Medical Center Information System).
Anesthetic management and procedural technique
No premedication was administered to any of the patients. In addition to noninvasive blood pressure monitoring, the electrocardiogram, arterial oxygen saturation, and partial pressure of end-tidal carbon dioxide were monitored for every patient. For patients undergoing the procedure under MAC, oxygen was routinely administered via a simple mask at the rate of 6 L/min. As a loading dose, 1.0 µg/kg of dexmedetomidine was injected for over a 15-minute periods, followed by a continuous infusion at a rate of 0.5 µg/kg/hr. Additionally, 1–2 mg of IV midazolam was infused to assist with the sedation. Remifentanil was also administered by target-controlled infusion at an effect site concentration of 0.3–1.0 ng/mL during the procedure. To maintain a deep sedation state in a patient, the bispectral index was monitored, and the target range was between 60 and 80. The TEE probe was inserted when a patient lost consciousness after the dexmedetomidine loading.
Patients undergoing GA were preoxygenated with 100% oxygen at a flow rate of 8 L/min and were administered with 2 mg/kg of propofol intravenously as an induction dose. After an injection of 0.6 mg/kg of rocuronium to produce the neuromuscular blockade, tracheal intubation was done, and a TEE probe was inserted. The anesthesia was maintained with 2% sevoflurane in a 50% oxygen and air mixture. After the procedure was completed, pyridostigmine mixed with glycopyrrolate was used as a reversal agent for the neuromuscular blockade, and the patient was extubated when the recovery from anesthesia was confirmed.
The procedural technique was not different between the two groups. Before beginning the procedure, an evaluation with TEE and balloon sizing was performed to decide on the size of the device to be used. The Amplatzer septal occluder (AGA Medical, Golden Valley, MN, USA) was used for the transcatheter closure procedure. After the procedure, transthoracic echocardiography was performed to confirm the position of the device and to monitor for the occurrence of complications (6).
Evaluation of the outcomes
The primary outcome was the overall complication rate that included the incidence of pulmonary, neurologic, cardiac, device-related, and other complications. The pulmonary complications monitored included the incidence of aspiration pneumonitis, laryngospasms, bronchospasms, pneumonia, acute respiratory distress syndrome, atelectasis, pneumothorax, and pleural effusion. The neurologic complications were defined as the occurrence of post-procedural delirium, cognitive dysfunction, stroke, or spinal cord ischemia. New-onset arrhythmia, ischemic heart disease, hemodynamic instability requiring the administration of cardiovascular medication or intervention, and incorrect position of the device were included among the monitored cardiac complications. Device-related complications were defined as embolization erosion, fracture, or thrombus formation of closing device that occurred from the day of the procedure to the present. Complications other than those that could be classified into the above categories, including vocal cord palsy or a sore throat, were defined as other complications.
The secondary outcomes were the procedure time, turnover time, and duration of hospital stay. The procedure time was defined as the duration of the time taken from the femoral venous puncture to the removal of the last catheter. The turnover time was defined as the duration of the time period from the moment the patient entered the operating room to the time point corresponding to their exit from the operating room. Additionally, we investigated the percentage of cases in which the patients were switched from MAC to GA during the procedure.
Statistical analysis
Continuous variables such as age, body mass index, hematocrit values, creatinine and albumin levels, the left atrium size, ASD size, left ventricular ejection fraction, procedure time, turnover time, and duration of hospital stay were presented as the mean ± standard deviation or medians with the interquartile range, and were analyzed using the t-test or Mann-Whitney rank-sum U test. Categorical variables such as the sex, American Society of Anesthesiologists classification, New York Heart Association classification, comorbidities, and complications encountered were presented as frequencies and percentages and assessed using the Pearson’s χ2 or Fisher’s exact test. Statistical analyses were conducted using SPSS (Version 21.0; SPSS Inc, Chicago, IL, USA). In all comparisons, P<0.05 was considered statistically significant.
Results
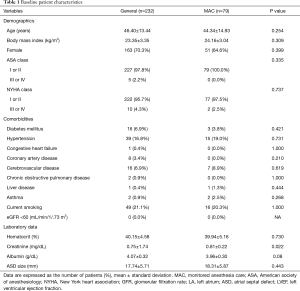
A total of 324 patients (MAC, 80 patients; GA, 244 patients) were involved in this study. After excluding 13 patients who underwent the procedure at the Pediatric Cardiology Department, a total of 311 patients were enrolled in the final analysis (Figure 1). The demographic data of all the study patients are shown in Table 1. Among the study cases, 79 cases were performed under MAC with deep sedation, and 232 cases were performed under GA. In the MAC group, two cases involved patients being switched from MAC to GA (2.5%), one of which was due to the onset of respiratory depression and the other was because of cardiac tamponade. The overall complication rate was not different between the two groups [25/232 (10.8%) in the GA group vs. 7/79 (8.9%) in the MAC group; P=0.830] (Table 2). The incidence of cardiac complications was not significantly different between the two groups [7/232 (3.0%) in the GA group vs. 3/79 (3.8%) in the MAC group; P=0.718]. In the GA group, cardiac complications including the onset of paroxysmal supraventricular tachycardia, atrioventricular block, bradycardia, right ventricle failure due to air embolism, and frequent premature ventricular contractions accompanied by chest discomforts occurred. In the MAC group, the onset of bradycardia, temporary cardiac arrest, and cardiac tamponade were recorded as cardiac complications. All patients with cardiac complications were treated as needed and discharged without active problems. There was only one pulmonary complication overall, which occurred in the GA group (ventilator weaning failure, pulmonary edema). Aspiration pneumonia did not occur in any patient. Neurologic complications also did not occur in the entire patient group of the study. There were no difference in the occurrence of intubation related complications (sore throat, vocal cord palsy, dysphagia) between the two groups [7/232 (3.0%) vs. 1/79 (1.3%); P=0.685]. The incidences of device-related complications did not show significant difference between two groups (0.4% vs. 0%, P=1.000). The procedure time and duration of hospital stay were not different between the groups. However, the turnover time was significantly shorter in the MAC group (55.87±12.89 vs. 50.90±15.50 min; P=0.005) (Table 2).
Full table

Full table
Discussion
In our present study, the outcomes of performing a percutaneous ASD device closure under MAC with deep sedation did not demonstrate any differences in the overall complication rate, which included pulmonary, neurologic, cardiac, and the other complications when compared to the outcomes of performing this procedure under GA. We also found that there were no significant differences in the procedure time and duration of hospital stay between the two groups; however, the turnover time was found to be shorter in the MAC group.
During this procedure, the patient is generally made to lie down in the supine position and the TEE probe should be inserted. Thus, the use of GA has been considered to be necessary for the protection of airway and comfort of the patient during the procedure (3). The onset of aspiration pneumonia is one of the feared complications that could arise during this procedure when the patient is sedated but not intubated. In a study that analyzed data of 100,359 patients undergoing colonoscopies, the incidence of aspiration was reported to be 0.14% when the procedure was performed under sedation by an anesthesia service (7). In another study involving 293 patients undergoing endoscopic submucosal dissections performed under sedation with propofol and remifentanil, the incidence of aspiration pneumonia was reported to be from 1.4% (in analgesia targeted light sedation group) to 5.2% (in moderate sedation with analgesic supplementation group) (8). In contrast, in our current study no aspiration was reported in any of the patients including in the MAC group, suggesting the safety of deep sedation with dexmedetomidine with regard to this potential complication. It is known that dexmedetomidine has a side effect that causes reduction of salivary secretion (9,10). The activation of the central alpha 2-receptor inhibits the central cholinergic activation and causes salivary gland vasoconstriction (11). It is also suggested that dexmedetomidine increases the expression of phosphodiesterase 4D, reducing the fluid secretion of the salivary glands (12). This anti-salivary effect of dexmedetomidine seems to have contributed to its protective effect against aspiration-related complications.
There have been some reports recently that have suggested that the ASD device closure can be performed safely and effectively under conscious sedation or deep sedation (4,5,13). In a retrospective cohort study which involved 197 children undergoing percutaneous ASD closures under deep sedation with spontaneous breathing, the success rate of the intervention was high (unsuccessful procedures, 5.7%), and the incidence of complications associated with sedation or the procedure in general was relatively low (no major cardiorespiratory complications; minor respiratory complications, 8%) (5). In a study involving 43 patients undergoing transcatheter ASD closures, Desai et al. (4) reported that conscious sedation using dexmedetomidine was a safe and effective technique with a procedure success rate of 93% and the incidence of complications occurring rarely resulting in a very low to no complication rate. Another study which involved 122 patients also reports that the procedure was performed safely (free of any complications, 91.0%) and successfully (implantation success, 98.4%) under conscious sedation, and the authors suggest that the procedure time observed in their study was significantly shorter than the duration of the procedures under GA reported in other literatures (13). Nevertheless, few studies have been conducted that have involved directly comparing the use of GA versus sedation in this procedure. Within our knowledge, our present study is the first to directly compare the outcomes of using GA and MAC to perform this procedure. Similar to other studies, the success rate of this procedure was high (procedural failure, GA, 0%; MAC, 1.3%), and the incidence of overall complications was low (GA, 10.8%; MAC, 8.9%), without significant differences observed between the two groups. Specifically, there was only one procedural failure associated with the MAC process, suggesting the feasibility and safety of this protocol in percutaneous ASD device closures.
Our current study has several limitations. As this was a retrospective study, it is possible that unexpected biases were involved. Although the two groups were not significantly different in their baseline characteristics, there could be confounding factors that were not detected but affected the outcomes of the study. We also cannot rule out selection bias because the choice of the method employed to induce anesthesia was made at the discretion of the anesthesiologist. Additionally, as this study was performed by reviewing electronic health records, it is possible that reports outlining the complications encountered could have been omitted or could not have been recorded. A randomized prospective study is warranted to compare the outcomes of anesthesia methods used to perform this procedure with minimal biases.
In conclusion, percutaneous ASD device closure under MAC with deep sedation showed comparable safety and efficiency to the procedure under GA. Thus, MAC with deep sedation can be considered as an alternative to GA for performing this procedure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of Asan Medical Center, the affiliated hospital of University of Ulsan, College of Medicine (No. 2017-0779). Written informed consent was waived by the institutional review board because of the minimal risk of this retrospective study.
References
- Masura J, Gavora P, Formanek A, et al. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 1997;42:388-93. [Crossref] [PubMed]
- Omeish A, Hijazi ZM. Transcatheter closure of atrial septal defects in children & adults using the Amplatzer Septal Occluder. J Interv Cardiol 2001;14:37-44. [Crossref] [PubMed]
- Calvert PA, Klein AA. Anaesthesia for percutaneous closure of atrial septal defects. Continuing Education in Anaesthesia Critical Care & Pain 2008;8:16-20. [Crossref]
- Desai PM, Umbarkar SR, Sarkar MS, et al. Conscious sedation using dexmedetomidine for percutaneous transcatheter closure of atrial septal defects: a single center experience. Ann Card Anaesth 2016;19:463-7. [Crossref] [PubMed]
- Hanslik A, Moysich A, Laser KT, et al. Percutaneous closure of atrial septal defects in spontaneously breathing children under deep sedation: a feasible and safe concept. Pediatr Cardiol 2014;35:215-22. [Crossref] [PubMed]
- Jang JY, Heo R, Cho MS, et al. Efficacy of 3D transoesophageal echocardiography for transcatheter device closure of atrial septal defect without balloon sizing. Eur Heart J Cardiovasc Imaging 2018;19:684-9. [Crossref] [PubMed]
- Cooper GS, Kou TD, Rex DK. Complications following colonoscopy with anesthesia assistance: a population-based analysis. JAMA Intern Med 2013;173:551-6. [Crossref] [PubMed]
- Yoo YC, Park CH, Shin S, et al. A comparison of sedation protocols for gastric endoscopic submucosal dissection: moderate sedation with analgesic supplementation vs analgesia targeted light sedation. Br J Anaesth 2015;115:84-8. [Crossref] [PubMed]
- Bischoff P, Kochs E. Alpha 2-agonists in anesthesia and intensive medicine. Anasthesiol Intensivmed Notfallmed Schmerzther 1993;28:2-12. [Crossref] [PubMed]
- Karhuvaara S, Kallio A, Salonen M, et al. Rapid reversal of alpha 2-adrenoceptor agonist effects by atipamezole in human volunteers. Br J Clin Pharmacol 1991;31:160-5. [Crossref] [PubMed]
- Moreira TS, Takakura AC, Menani JV, et al. Activation of central alpha2-adrenoceptors mediates salivary gland vasoconstriction. Arch Oral Biol 2013;58:167-73. [Crossref] [PubMed]
- Ji M, Park CK, Lee JW, et al. Two phase modulation of NH4+ entry and Cl−/HCO3- exchanger in submandibular glands cells by dexmedetomidine. Front Physiol 2017;8:86. [Crossref] [PubMed]
- Lipiec P, Miskowiec D, Peruga JZ, et al. Conscious sedation for transcatheter implantation of atrial septal occluders with two- and three-dimensional transoesophageal echocardiography guidance - a feasibility and safety study. Kardiol Pol 2018;76:406-12. [PubMed]