Routine practice in mechanical ventilation in cardiac surgery in Italy
Introduction
Management of mechanical ventilation is among the most important skills for a modern anesthesiologist. Securing the airway and providing controlled or assisted mechanical ventilation are fundamental requirements for safe delivery of anesthesia. In recent decades, much progress has been made in protective lung strategy during surgery, including low tidal volume (TV), low plateau and driving pressure, recruitment maneuvers (RMs), and adequate positive end-expiratory pressure (PEEP) (1-3).
This is especially essential in cardiac surgery since many factors can contribute to lung injury, including general anesthesia itself, cardiopulmonary bypass (CPB), blood transfusions, cardiac failure, and diaphragmatic dysfunction—all of which increase the risk of postoperative pulmonary complication (PPC), as defined by Abbott et al. (4) in up to 25% of patients after surgery (5-7). Nevertheless, evidence regarding the best way for lung protective ventilation is still lacking.
Based on the assumption that there is no uniformity in the ventilatory management of the cardiac surgery patient in Italian hospitals, we conducted a survey to investigate and understand the current clinical practices in our country, Italy.
Methods
From April 2017 to April 2018, we identified 69 centers performing adult cardiac surgery, and an electronic 32-item questionnaire was sent to the 56 centers (81.2%) that accepted our invitation to take part in the survey. The questionnaires were first sent in April 2017, followed by monthly reminders addressed specifically to non-responders until April 2018. The dataset supporting the conclusions of this article is included within the article and Supplementary file 1. Main data graphs are included in http://fp.amegroups.cn/cms/jtd.2019.03.04-1.pdf, http://fp.amegroups.cn/cms/jtd.2019.03.04-2.pdf.
Our work was endorsed by the Italian Society of Anesthesia, Analgesia, Resuscitation and Intensive Care (SIAARTI). We sent an e-mail invitation to all members of the SIAARTI Study Group on Cardiothoracic and Vascular Anesthesia. The centers performing cardiac surgery in Italy were identified through the Italian Society of Cardiac Surgery (SICCH) website (http://www.sicch.it/), and further information for Cardiothoracic intensive care units (ICUs) were obtained from hospital websites and personal contacts. There were no specific inclusion criteria for the centers. All participating anesthesiologists were informed about the aims of the study. If the chiefs of these ICUs did not respond to our first e-mail, they were personally re-contacted by e-mail and invited to participate.
Respondents were asked to indicate one (or more, when necessary) answer to each question using the Google Forms online platform. The survey could not be submitted unless completed since all questions were flagged as mandatory. The respondents were unable to review their answers. All information collected was protected, and no personal contact information was accessible to third parties.
A case report form (CRF) (Supplementary file) consisted of 31 questions regarding both intra- and post-operative issues and was designed to evaluate each participant. When more than one questionnaire was returned by the same hospital (i.e., more than one physician from the same hospital answered the first e-mail), divergences between answers given in the questionnaires were resolved by contacting the head of that department. In this survey, this happened for 17 centers.
The questionnaire sought to investigate the most important aspects of the ventilation setting and use of lung protection strategies in cardiac surgery, both in the intraoperative and postoperative periods. All phases were analyzed, with special attention given to the complex phase of CPB. Three researchers independently developed the items of the survey before the final selection and collection via the questionnaire.
By taking part in the survey, each physician authorized the use of the data recorded in the questionnaire. Informed consent for publication was obtained at the time of participation. The consent manifestation, utilization, and communication of the data collected were performed according to the European General Data Protection Regulation (2016/679, in place as of May 25, 2018). Due to the study design, no ethical approval was required. Specific data regarding individual patients was not collected and therefore remained completely anonymous. This research was carried out in compliance with the Declaration of Helsinki.
Statistical analyses were performed using IBM SPSS Statistics 25.0 (IBM, Armonk, NY, USA) and GraphPad Prism, version 6.01 for Windows (GraphPad Software, San Diego, CA, USA). A descriptive statistical analysis was carried out.
Results
A complete list of the answers to the questionnaire is summarized in Supplementary file 1. Further analyzes were made by stratifying the centers by volume and teaching and not-teaching centers; the results of these analyzes are reported respectively in the http://fp.amegroups.cn/cms/jtd.2019.03.04-1.pdf, http://fp.amegroups.cn/cms/jtd.2019.03.04-2.pdf.
Center characteristics
The questionnaire was completed and returned by 81.2% of the participant centers, 66.1% of which were non-teaching hospitals, while 33.9% were teaching hospitals. A complete list of the responding centers is available in http://fp.amegroups.cn/cms/jtd.2019.03.04-3.
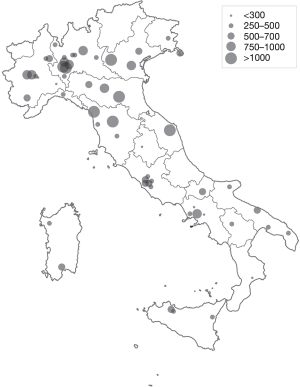
High patient flow (>1,000 cardiac surgery procedures per year, with or without CPB) was reported at 7 centers (12.5%). Eight centers (14.3%) reported 750–1,000 procedures per year; 12 centers (21.4%) reported 500–750, 23 (41.1%) centers performed 250–500 procedures per year; and 6 centers (10.7%) performed <250 procedures per year (Figure 1).
Type of ventilation in operating room
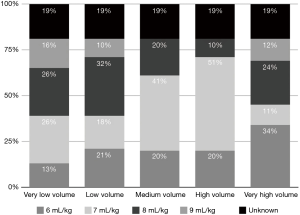
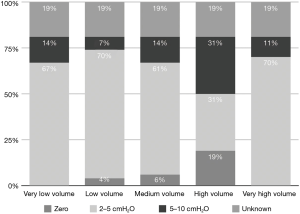
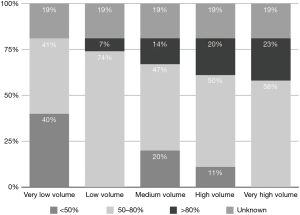
Low TV ventilation (regardless of the method of ventilation chosen, either pressure-controlled or volume-controlled intermittent positive-pressure ventilation) was used in 91.1% of centers, with TVs of 6 mL/kg (26.8%), 7 mL/kg (33.9%), and 8 mL/kg (30.4%), while a TV of 8–10 mL/kg was used in 8.9% of the centers (Figure 2). The TV was calculated on the ideal body weight in 57% of the centers and on the real body weight in the other 43%. An average PEEP of 3–5 cmH2O was used in 76.8% of centers, and 5–10 cmH2O in another 16.1%. Zero PEEP was used in 7.1% of centers. The “best PEEP” was assessed in only 1.8% of the centers intraoperatively (Figure 3). A definition of best PEEP was not provided by the questionnaire since there are many methods to assess the best PEEP in clinical practice. The results simply describe the attempt to set a best PEEP value. In 60.7% of the centers, fraction of inspired oxygen (FiO2) was between 50–80% at the end of surgery. In the other centers, patients were managed with an FiO2 of less than 50%. We documented a wide range of FiO2 applied during weaning from CPB: 73.2% of the centers applied an FiO2 of 50–80%, while 14.3% used an FiO2 of more than 80%. The 39% of centers use a FiO2 <50% during surgery (Figure 4) while only 12.5% of centers used an FiO2 of <50% at the weaning from CPB.
Ventilation was generally stopped during CPB (75% of the total centers). Continuous positive airway pressure (CPAP) was applied in 16.1% of the total centers, while only 8.9% did not stop ventilation during CPB (Figure 5).
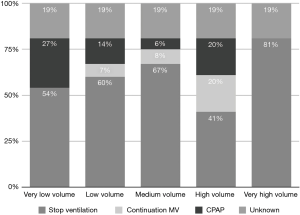
In centers where ventilation was continued during CPB, a CPAP of 5–8 cmH2O was used in 71.4% of centers, with <5 cmH2O in the other 21.4% of centers; a CPAP higher than 8 cmH2O was reported in only 7.1% of centers. In those centers, TV and PEEP values varied from a very low TV of 2–3 mL/kg in 73.3% of the centers to a 3–5 mL/kg TV in 26.7%. A PEEP level of 3–5 cmH2O was used in 52.6% of the centers, while a PEEP >5 cmH2O was used in 5.3%. A PEEP of 1–3 cmH2O was used in 31.6%, with zero PEEP in the remaining 10.5%.
When ventilation was stopped, almost one-third of centers disconnected patients from the anesthesia circuit.
RMs were used at 43 centers (76.8%) and were performed manually at 38 centers (88% of the centers performing RMs).
Ventilation during patient transport to the ICU
In 12.8% of centers, a fast-track protocol was in use, defined as “extubation carried out in the operating room.” When mechanical ventilation was continued in the ICU, manual ventilation was used in 87.5% of centers, while 12.5% used a portable mechanical ventilator.
Type of ventilation in ICU
On arrival in the ICU, the most widely used type of ventilation was synchronized intermittent mandatory ventilation (SIMV) (either pressure-controlled or volume-controlled SIMV; 41.1% of centers), followed by pressure support (39.3%), bilevel positive airway pressure (BIPAP) (14.3%), volume-controlled mechanical ventilation (VC-CMV) (3.6%), and pressure-controlled mechanical ventilation (PC-CMV) (1.8%).
Once back in the ICU, a TV of 8 mL/kg was used in 32.1% of centers, followed by 7 mL/kg in 32.1%, 6 mL/kg in 25%, and 8–10 mL/kg in 10.7%. PEEP was used in all centers, with values set between 3–5 cmH2O (66.1% of centers), 6–8 cmH2O (30.4%), and 8–10 cmH2O (3.6%). In only 23.2% of the centers was “best PEEP” calculated during the ICU stay. We did not specifically ask the method used to asses a best PEEP, since there is no consensus about this important topic among intensive care physicians (8) we only asked if a method was used or the PEEP was simply chosen “clinically”, without respiratory mechanics measurements. Respiratory RM were used in 53.6% of centers before extubation. In centers where a fast-track approach was used, a myorelaxant antagonist was administered before extubation in 28.6% of centers, with 65.7% using neostigmine vs. 34.3% using sugammadex.
Before the discharge from the intensive care unit non-invasive ventilation (NIV) was never applied in 32.1% of the centers, but it was used in 21.5% of centers for selected patients and in 46.4% of centers only for patients with postoperative complications.
Discussion
The main result of this survey is the lack of a univocal approach among Italian cardiac surgery centers according to the most recent evidence on the best strategy for lung protection in cardiac surgery, a result similar to other surveys (9). However, to our knowledge, we now report this issue in the literature for the first time.
In fact, it is known that most data on protective ventilation come from the experiences of other major surgical centers and mixed ICUs, suggesting the use of low TV, PEEP (10), and, generally speaking, reduction of ventilator-induced lung injury. Moreover, it is also important to calculate the TV on the ideal weight and not on the real one, especially in cases of patients who are severely overweight and obese, where using the increased actual weight to calculate TV targets will overestimate the target TV and expose these patients to harmful volutrauma and barotrauma (11). Two recent meta-analyses showed how protective ventilatory strategies could help reduce PPCs during general anesthesia, as well as possibly shorten the length of a hospital stay (12,13). However, there is still no significant evidence in the literature regarding the most suitable ventilatory strategy to use in cardiac surgery.
Nonetheless, lung damage in cardiac surgery seems to follow the main pathways of barotrauma (high transpulmonary pressure), atelectasis (lack of adequate lung recruitment, particularly if ventilation is stopped during CPB), and inflammation (both ventilation-induced and because of CPB itself) (5,14,15). High plateau pressures and driving pressures (generating high transpulmonary pressures) are likely to damage lung parenchyma in cardiac surgery as well as in general surgery. Moreover, cardiac surgery can present further possible sources of lung injury, such as the inflammatory response induced by CPB (6), complete collapse of lung parenchyma caused by interruption of ventilation during CPB (5,16,17), injury induced by blood transfusion (18), production of proinflammatory cytokines linked to CPB-related myocardial damage, and the production of free radicals following reperfusion of myocardial tissue after CPB (19,20).
In this context, lung-protecting ventilation strategies—including the use of low TVs, RMs, adequate FiO2, avoiding hyperoxia (i.e., absorption atelectasis), and NIV or CPAP during postoperative ICU stay where indicated—are thought to play key roles in lung protection. However, there is no high-quality evidence available in the context of cardiac surgery and cardiac ICU. In this way, the lack of consensus in the current practice is not surprising. New research in the field of mechanical ventilation in cardiac surgery is warranted.
FiO2 management
Regarding FiO2 management, a recent review investigated the effects of oxygen fraction, concluding that moderate hyperoxia (50−80% FiO2) is potentially beneficial owing to the reduced incidence of surgical-site infections and the absence of demonstrated clinical drawbacks (21). However, conflicting opinions are still present in the literature (22-24). From a pathophysiological point of view, we can say that hemoglobin saturation higher than 100% is not possible, and the fraction of oxygen not bound to hemoglobin carries an insignificant percentage of oxygen delivery at 1 atm. Therefore, it is questionable to keep partial oxygen pressure higher than needed to saturate hemoglobin.
A recent trial compared moderate hyperoxic targets to near-physiological oxygen targets during and after coronary artery bypass surgery, with myocardial damage as a primary end-point (25). The use of a normoxemic strategy did not affect the incidence of myocardial damage, nor did it influence secondary outcomes (such as cardiac index, systemic vascular resistance index, serum creatinine and lactate. A recent meta-analysis based on 12 randomized controlled trials (RCTs) showed the minimal effect of hyperoxia on organ dysfunction, length of hospital stay, and mortality in adult cardiac surgery (26). An ongoing study is currently comparing the increase in serum creatinine in hyperoxygenated patients (FiO2 of 100%) and those undergoing physiological oxygenation (27).
High oxygen concentrations are associated with the development of absorption atelectasis (28), which can lead to the onset of PPC. At present, there is no strong evidence as to how a high FiO2 might prevent infection in the surgical site and further the development of hyperoxia-induced atelectasis predisposing to postoperative pulmonary infections. There is currently no strong evidence on what might be considered best treatment, with further studies required.
The use of RMs and PEEP could reduce the incidence of atelectasis. Although many studies agree on the usefulness of RMs, a best method has yet to be defined, as does the ideal pressure to reach during the maneuver (28). A recent study carried out during the postoperative period investigated whether an intensive alveolar recruitment strategy yields better results than does a moderate strategy. All patients were ventilated with low-to-moderate TV. The intensive strategy, involving three cycles of lung inflation (60 seconds each), consisting of PEEP of 3 cmH2O, pressure-controlled ventilation, driving pressure of 15 cmH2O, respiratory rate of 15/min, inspiratory time of 1.5 seconds, and FiO2 of 40% was better at preventing PPCs and able to reduce the incidence of severe pulmonary complications (29).
Ventilation during CPB
Although no unequivocal consensus currently exists, there are three options for the management of ventilation during CPB:
- CPAP: various studies used CPAP with pressures between 5–15 cmH2O and showed different results;
- Mechanical ventilation: low TV frequency ventilation as postoperative oxygenation showed a positive effect on secondary outcomes;
- Lung rest: this seems to be the best option for the surgeon. however, the studies investigated in two systematic reviews showed no significant differences in surgical times compared to experimental arms (27,30).
None of the studies investigated in the two reviews showed damage caused by intra-CPB ventilation, nor by pre- or post-CPB protective ventilation (6,31,32). However, not every study involved human subjects or cardiac surgery (33).
There is no definitive evidence in the literature showing the superiority of a specific method of ventilation; further studies are required.
Postoperative NIV
NIV can be used both to prevent and to treat PPCs. A recent study by Olper et al. (34) promotes the efficacy of early NIV applications in the cardiac surgery ward, with improved oxygenation in patients who develop hypoxemic acute respiratory failure after discharge from the ICU. It seems that even high-flow nasal oxygen gives results comparable to classic NIV (35).
Limitations of the study
A strong limitation of this study is the differences in clinical practices among anesthesiologists at the same center. When anesthesiologists at the same center gave significantly different answers, the head of the center was contacted to clarify the data.
As a general limitation of the survey, we cannot verify whether what a single physician declares is truly correct and reflects the current practice in his or her center. Further, if a survey uses different words or phrases from those in a particular clinical practice, the results of that survey could have a different story to tell. Moreover, we did not receive answers from all the centers in Italy; therefore, we cannot assume our data to be conclusive. In the non-responding centers, there may be differences in clinical practice from the responses we were able to collect.
Different physicians can have varying opinions regarding management of the same patient based on their personal experience. However, our questionnaire was designed to investigate procedures that generally respected the established protocol of each specific center.
Conclusions
This survey describes the current state of Italian ventilation management during cardiac surgery and shows that there is still some heterogeneity among ventilation settings, in particular, during CPB (although protective ventilation in other surgeries is widespread).
Focusing on current scientific evidence, standards of care need to be established concerning ventilation of patients during cardiac surgery at Italian centers. Further research is needed to investigate the methods of protective ventilation, as these techniques have already produced important results in other contexts.
Supplementary
A complete list of the answers to the questionnaire
Acknowledgements
We would like to thank all the centers of cardiothoracic surgery in Italy that contributed to this survey giving us the information we collected. We also want to thank Michael John, of the Vita-Salute University, for English language editing of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This research was carried out in compliance with the Declaration of Helsinki. Informed consent for publication was obtained at the time of participation.
References
- Neto AS, Hemmes SNT, Barbas CSV, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 2016;4:272-80. [Crossref] [PubMed]
- Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428-37. [Crossref] [PubMed]
- Zamani MM, Najafi A, Sehat S, et al. The effect of intraoperative lung protective ventilation vs conventional ventilation, on postoperative pulmonary complications after cardiopulmonary bypass. J Cardiovasc Thorac Res 2017;9:221-8. [Crossref] [PubMed]
- Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018;120:1066-79. [Crossref] [PubMed]
- Apostolakis E, Filos KS, Koletsis E, et al. Lung dysfunction following cardiopulmonary bypass. J Card Surg 2010;25:47-55. [Crossref] [PubMed]
- Bignami E, Guarnieri M, Saglietti F, et al. mechanical ventilation during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2016;30:1668-75. [Crossref] [PubMed]
- Bignami E, Guarnieri M, Saglietti F, et al. Diaphragmatic dysfunction followingcardiac surgery: is there a role for pulmonary ultrasound? J Cardiothorac Vasc Anesth 2018;32:e6-7. [Crossref] [PubMed]
- Hubmayr RD, Malhotra A. Still looking for best PEEP. Anesthesiology 2014;121:445-6. [Crossref] [PubMed]
- Kim SH, Na S, Lee WK, et al. Application of intraoperative lung-protective ventilation varies in accordance with the knowledge of anaesthesiologists: a single-centre questionnaire study and a retrospective observational study. BMC Anesthesiol 2018;18:33. [Crossref] [PubMed]
- Sutherasan Y, Vargas M, Pelosi P. Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Crit Care 2014;18:211. [Crossref] [PubMed]
- Rehder KJ, Turner DA. Actual versus ideal body weight: the devil is in the details. Respir Care 2018;63:1189-90. [Crossref] [PubMed]
- Yang D, Grant MC, Stone A, et al. A meta-analysis of intraoperative ventilation strategies to prevent pulmonary complications: is low tidal volume alone sufficient to protect healthy lungs? Ann Surg 2016;263:881-7. [Crossref] [PubMed]
- Hemmes SN, Serpa Neto A, Schultz MJ. Intraoperative ventilatory strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol 2013;26:126-33. [Crossref] [PubMed]
- Babik B, Asztalos T, Peták F, et al. Changes in respiratory mechanics during cardiac surgery. Anesth Analg 2003;96:1280-7. [Crossref] [PubMed]
- Wynne R, Botti M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: clinical significance and implications for practice. Am J Crit Care 2004;13:384-93. [PubMed]
- Young RW. Hyperoxia: a review of the risks and benefits in adult cardiac surgery. J Extra Corpor Technol 2012;44:241-9. [PubMed]
- Müller H, Hügel W, Reifschneider HJ, et al. Lysosomal enzyme activity influenced by various types of respiration during extracorporeal circulation. Thorac Cardiovasc Surg 1989;37:65-71. [Crossref] [PubMed]
- Fransen E, Maessen J, Dentener M, et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest 1999;116:1233-9. [Crossref] [PubMed]
- Romagnoli S, Becatti M, Bonicolini E, et al. Protective ventilation with low fraction of inspired oxygen and radicals of oxygen production during general anaesthesia. Br J Anaesth 2015;115:143-4. [Crossref] [PubMed]
- Corral-Velez V, Lopez-Delgado JC, Betancur-Zambrano NL, et al. The inflammatory response in cardiac surgery: an overview of the pathophysiology and clinical implications. Inflamm Allergy Drug Targets 2015;13:367-70. [Crossref] [PubMed]
- Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care 2013;58:123-41. [Crossref] [PubMed]
- Wetterslev J, Meyhoff CS, Jørgensen LN et al. The effects of high perioperative inspiratory oxygen fraction for adult surgical patients. In: Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd.; 1996:991.
- Stall A, Paryavi E, Gupta R, et al. Perioperative supplemental oxygen to reduce surgical site infection after open fixation of high-risk fractures: a randomized controlled pilot trial. J Trauma Acute Care Surg 2013;75:657-63. [Crossref] [PubMed]
- Bignami E, Saglietti F, Girombelli A, et al. Preoxygenation during induction of anesthesia in non-critically ill patients: a systematic review. J Clin Anesth 2019;52:85-90. [Crossref] [PubMed]
- Smit B, Smulders YM, de Waard MC, et al. Moderate hyperoxic versus near-physiological oxygen targets during and after coronary artery bypass surgery: a randomised controlled trial. Crit Care 2016;20:55. [Crossref] [PubMed]
- Heinrichs J, Lodewyks C, Neilson C, et al. The impact of hyperoxia on outcomes after cardiac surgery: a systematic review and narrative synthesis. Can J Anaesth 2018;65:923-35. [Crossref] [PubMed]
- Lopez MG, Pretorius M, Shotwell MS, et al. The risk of oxygen during cardiac surgery (ROCS) trial: study protocol for a randomized clinical trial. Trials 2017;18:295. [Crossref] [PubMed]
- Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care 2015;60:609-20. [Crossref] [PubMed]
- Costa Leme A, Hajjar LA, Volpe MS, et al. Effect of intensive vs. moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: a randomized clinical trial. JAMA 2017;317:1422-32. [Crossref] [PubMed]
- O’Brien J. Absorption atelectasis: incidence and clinical implications. AANA J 2013;81:205-8. [PubMed]
- Lellouche F, Delorme M, Bussières J, et al. Perioperative ventilatory strategies in cardiac surgery. Best Pract Res Clin Anaesthesiol 2015;29:381-95. [Crossref] [PubMed]
- Chi D, Chen C, Shi Y, et al. Ventilation during cardiopulmonary bypass for prevention of respiratory insufficiency: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6454. [Crossref] [PubMed]
- Ferrando C, Soro M, Belda FJ. Protection strategies during cardiopulmonary bypass: ventilation, anesthetics and oxygen. Curr Opin Anaesthesiol 2015;28:73-80. [Crossref] [PubMed]
- Olper L, Bignami E, Di Prima AL, et al. continuous positive airway pressure versus oxygen therapy in the cardiac surgical ward: a randomized trial. J Cardiothorac Vasc Anesth 2017;31:115-21. [Crossref] [PubMed]
- Stéphan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs. noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA 2015;313:2331-9. [Crossref] [PubMed]