Hydrogen gas inhalation ameliorates lung injury after hemorrhagic shock and resuscitation
Introduction
Post-traumatic hemorrhagic shock is the second highest cause of early death (1-3). Therefore, to reduce early mortality from post-traumatic shock, aggressive treatments, such as resuscitation, should be considered. However, there have been reports suggesting that the extent of tissue damage from ischemic-reperfusion injury due to hemorrhagic shock may be exacerbated when oxygen is reintroduced into the tissue after resuscitation (4). In other words, hemorrhagic shock and resuscitation (HSR), which are commonly observed in trauma patients, may cause inflammatory reactions in the lung parenchyma, including various organs, as well as acute lung injury, which may be triggered by HSR, and increase complications of trauma patients that can ultimately lead to death (5,6).
Reperfusion injury may be directly related to the formation of reactive oxygen species (ROS), damage of vascular endothelial cells, increased vascular permeability, and activation of neutrophils, platelets, cytokines, and complement systems (6). Among these, ROS is known to be the most important cause of reperfusion injury.
Antioxidants responsible for the inhibition and elimination of ROS have recently been introduced. Medical gases, such as hydrogen (H2), oxygen (O2), nitrous oxide (N2O), carbon monoxide (CO), nitrogen oxide (NO), and hydrogen sulfide (H2S), have been identified as these antioxidants (7). H2 in particular have been shown to have distinct characteristics, including its antioxidative effect on specific ROS and excellent diffusion capacity (7,8). The purpose of this study was to investigate the protective effect of 2% inhaled hydrogen gas on lung injury after HSR.
Methods
Animals
All surgical procedures and animal care were carried out in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, as well as the Guidelines and Policies for Swine Survival Surgery, provided by the Institutional Animal Care and Use Committee of the Ajou University Health System. Rats weighing 300–500 g (DBL, Chungcheongbuk-do, Korea) were used as experimental animals and were divided into three groups: the sham group, HSR group, and H2/HSR group.
HSR protocol
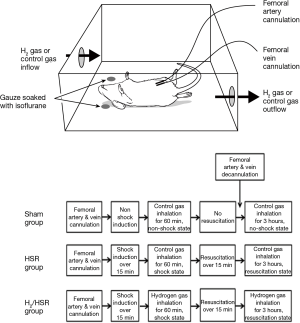
All rats were anesthetized with isoflurane-soaked gauzes, and the left femoral artery and femoral vein were exposed in aseptic condition. The polyethylene tube containing heparin was then cannulated into the femoral artery to acquire the mean arterial pressure during the experiment and into the femoral vein to extract blood while inducing hemorrhagic shock and administer blood and fluid during resuscitation. Prior to inducing hemorrhagic shock, blood pressure was measured in both the HSR and H2/HSR groups, via the femoral artery, to determine the reference mean arterial pressure. In the HSR and H2/HSR groups, hemorrhagic shock was induced by extracting the blood with heparin-containing syringe (10 units/mL) through the femoral vein cannulation for a period of 15 minutes until the mean arterial pressure reached 30 mmHg. These hemorrhagic shock conditions lasted for 60 minutes, during which, blood was further extracted or reintroduced through the femoral vein cannulation to maintain a mean arterial pressure between 25 and 35 mmHg. After 60 minutes, resuscitation was performed by injecting blood and fluid through the femoral vein over a period of 15 minutes until the mean arterial pressure reached the reference pressure. During the experiment, spontaneous breathing was maintained, electric blanket was used to maintain body temperature, and electrocardiogram and body temperature were continuously monitored. After resuscitation, the cannulation tube inserted into the femoral artery and femoral vein was removed, and the femoral wound was sutured. In the sham group, the cannulation tube was inserted for the same duration as the HSR and H2/HSR groups; after removing the cannulation tube, the femoral wound was sutured.
Two percent hydrogen gas and control gas composition and administration
The 2% hydrogen gas was composed of H2 (2%), O2 (21%), and nitrogen (N2) (77%); the control gas was composed of O2 (21%) and N2 (79%) (Praxair, Dongtan-si, Korea). Experimental rats were placed in a sealed plexiglas chamber with gas inlet and outlet lines, and 2% hydrogen gas was injected into the plexiglas chamber at a rate of 4 L/min. Inhalation of the hydrogen gas and control gas was performed for 1 hour after inducing hemorrhagic shock and for 3 hours after resuscitation. Hydrogen gas was inhaled only in the H2/HSR group, and control gas was inhaled in the sham and HSR groups (Figure 1).
Sacrifice of experimental rats and tissue extraction
After the experiment, rats were anesthetized with isoflurane, and the abdomen was incised to extract the blood from the abdominal aorta. During this procedure, normal saline was injected through the inferior vena cava until the blood of the abdominal aorta became transparent; at this point, lung tissue was extracted and immediately frozen in liquid nitrogen and stored at −80 °C until use. Mice died naturally during this process.
Arterial blood gas analysis
After HSR, arterial blood was extracted from the abdominal aorta. The following parameters were measure and compared between the groups via arterial blood gas analyses: blood lactate, PO2, and PCO2.
Histologic examination
Hematoxylin & Eosin (H&E) staining and histologic examination were performed to confirm the infiltration of inflammatory cells into the lungs. Lung tissue was fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned. After deparaffinization, the sections of lung tissue were stained with H&E and examined using an optical microscope.
Measurement of neutrophil infiltration into the lung tissue
Myeloperoxidase (MPO) activity was measured using colorimetric MPO assay kit (BioVision, CA, USA) to evaluate neutrophil infiltration into the lung tissue.
Measurement of proinflammatory mediators
Reverse transcription polymerase chain reaction (RT-PCR) was used to measure proinflammatory mediators. The expression of IL-1β, IL-6, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), intercellular adhesion molecule-1 (ICAM-1), and chemokine (C-C motif) ligand 2 (CCL2) were measured.
Statistical analysis
All statistical results were expressed as the mean value ± standard error of measurement (SEM). Parametric data were analyzed using one-way analysis of variance (ANOVA). Comparison of significant interactions and main effects for all statistical tests were performed using Bonferroni’s post hoc test. All statistical analyses were performed using SPSS version 24 (IBM Corp. Released 2016.IBM SPSS Statistics for Window, Version 24.0. Armonk, NY, USA: IBM Corp.). A P value of less than 0.05 was considered statistically significant.
Results
Arterial blood gas analyses and histologic examination
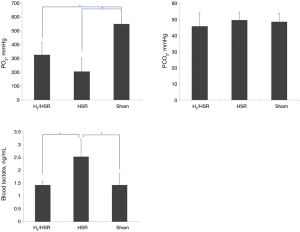
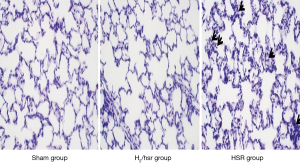
The PO2 values in the HSR and H2/HSR groups were lower than those in the sham group, but without statistical significance (Figure 2, Table 1). The PCO2 levels did not show statistically significant differences among the all groups. There was no statistically significant difference in the blood lactate levels between the sham group and the H2/HSR group. However, the HSR group had a significantly higher blood lactate level than the Sham and H2/HSR groups (Table 1). Infiltration of inflammatory cells into the lung tissues was more frequently observed in the HSR group than in the sham and H2/HSR groups (Figure 3).

Full table
Measurement of MPO activity and proinflammatory mediators
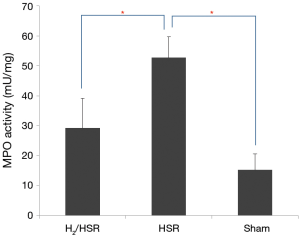
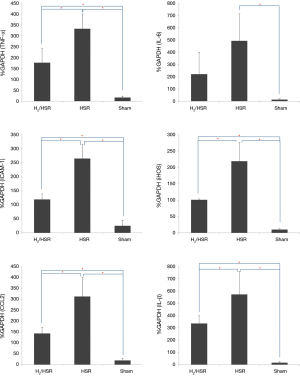
MPO activity was significantly different among the three groups; it was the highest in the HSR group, followed by the H2/HSR group and the sham group, in that order (Figure 4, Table 2). Regarding the proinflammatory mediators in lung tissue, all mediators, except for IL-6, were significantly different among the three groups. These mediators were most commonly observed in the HSR group, followed by the H2/HSR group and the sham group, in that order. In the case of IL-6, there was no statistically significant difference between the sham group and the H2/HSR group; however, it was significantly lower in the sham group and H2/HSR group compared with the HSR group (Figure 5, Table 3).

Full table

Full table
Discussion
In this study, we demonstrated that 2% hydrogen gas inhalation may play an important role in reducing inflammatory mediators in lung injury after HSR. The lung is the main target organ of inflammatory mediators after HSR. These inflammatory mediators cause acute lung injury that can eventually result in death. The most important substance in this inflammatory reaction is ROS, which is known to affect various organs, including the lungs (9). Treatment of ROS includes the secretion of antioxidants, such as glutathione peroxidase, and superoxide dismutase (SOD), in the body, as well as supplementation in vitro, such as hydrogen gas. However, studies on the role and function of antioxidants injected in vitro have not been conducted sufficiently, and this study shows that inhaled hydrogen gas may reduce inflammatory mediators.
According to our arterial blood gas analyses, there was a lower PO2 level in both the H2/HSR group and HSR group when compared with the sham group. This finding suggests that HSR causes lung injury and loss of pulmonary function. Moreover, our analyses showed that PCO2 was not significantly different among the three groups. The PCO2 level is known to be elevated toward the end stage of lung injury in patients with acute respiratory distress syndrome (10). Given that this experiment examined acute lung injury, the arterial blood PCO2 levels were not different between the groups. Blood lactate levels were higher in the HSR group than in the sham group and H2/HSR group. Increased blood lactate is known to be a strong predictor of multiple organ damage and mortality in critically ill patients. Moreover, blood lactate levels in patients with acute respiratory distress syndrome are known to increase in proportion to the severity of pulmonary disease (11). Therefore, elevated blood lactate in the HSR group indirectly suggests that there may be greater lung injury in the HSR group compared with the sham and H2/HSR groups.
Large infiltration of neutrophils into the lungs indicates acute lung injury. MPO activity is a direct measurement of the degree of neutrophil infiltration into the lungs and an indirect measurement of lung injury (12). We showed that MPO activity was lower in the H2/HSR group than in the HSR group. In other words, 2% hydrogen gas—to some degree—inhibited neutrophil infiltration and minimized the degree of lung injury.
A variety of inflammatory mediators, such as proinflammatory transcription factors and proinflammatory cytokines, are known to be involved in ischemic organ damage. Among these, TNF-α and IL-1β are considered as initiator cytokines that initiate stepwise activations of other mediators, such as activation of other cytokines, release of prostaglandin, and induction of chemotaxis of leukocytes due to inflammation (13). Therefore, as shown in this study, a reduction of TNF-α and IL-1β by 2% hydrogen gas inhalation may ultimately reduce the incidence of lung injury from the onset of resuscitation.
It is known that ICAM-1 induces neutrophil infiltration into the lungs and CCL2 attracts monocytes into the lungs (14,15). In this study, ICAM-1 and CCL2 levels were higher in the HSR group than in the H2/HSR group. This suggests that neutrophils and monocytes were infiltrated more frequently in the HSR group, hence greater progression of lung injury.
Although IL-6 may contribute to acute lung injury as an inflammatory mediator, it is known that IL-6 has an anti-inflammatory effect that can suppress the development of lung injury, depending on the type of receptors (16). However, IL-6 is elevated in all patients with acute respiratory distress syndrome or at risk of acute respiratory distress syndrome due to infectious, traumatic, or inflammatory diseases, and such elevated IL-6 level is associated with a higher risk of death (17). Thus, elevated IL-6, as a biomarker for pulmonary injury, can be used to predict the extent of lung damage in the HSR group. However, a definitive conclusion about whether elevated IL-6 contributes to lung injury remains unclear.
iNOS has been reported to have different effects depending on the stage of lung injury. In the early stages of pulmonary injury, iNOS has been shown to play a proinflammatory role, whereas in the late stages, it has been associated with inflammation suppression (18). In this study, iNOS, which was increased in the HSR group, behaved as a proinflammatory mediator, given that this study examined acute, early stage lung injury.
This study has the following limitation to consider. Although the protective effect of 2% inhaled hydrogen gas was demonstrated on post-HSR lung injury, the advantages of hydrogen gas relative to other medical gases were not evaluated. To address this, a future comparative study of hydrogen gas versus other medical gases will be necessary.
In conclusion, inhalation of 2% hydrogen gas after HSR can minimize the extent of lung injury by inducing a decrease in the MPO activity—and consequently inhibiting the infiltration of neutrophils into the lungs—and by inducing a decrease in the infiltration of inflammatory cells into the lung tissues—and consequently suppressing the formation of proinflammatory mediators, such as IL-1β, TNF-α, iNOS, ICAM-1 and CCL2. Although it has not been applied in clinical practice, the administration of hydrogen gas through a ventilator, nasal cannula, or simple inhalation in traumatic hemorrhagic patients is expected to reduce the extent of lung injury.
Acknowledgements
The authors would like to thank Dong-Su Jang, MFA, (Medical Illustrator) for his help with the illustrations.
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2012R1A1A2008312).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All surgical procedures and animal care were carried out in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, as well as the Guidelines and Policies for Swine Survival Surgery, provided by the Institutional Animal Care and Use Committee of the Ajou University Health System.
References
- Shackford SR, Mackersie RC, Holbrook TL, et al. The epidemiology of traumatic death. A population-based analysis. Arch Surg 1993;128:571-5. [Crossref] [PubMed]
- Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma 1995;38:185-93. [Crossref] [PubMed]
- Stewart RM, Myers JG, Dent DL, et al. Seven hundred fifty-three consecutive deaths in a level I trauma center: the argument for injury prevention. J Trauma 2003;54:66-70; discussion 70-1. [Crossref] [PubMed]
- Ferrari RS, Andrade CF. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid Med Cell Longev 2015;2015:590987. [Crossref] [PubMed]
- Mura M, Andrade CF, Han B, et al. Intestinal ischemia-reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock 2007;28:227-38. [Crossref] [PubMed]
- Zimmerman BJ, Granger DN. Mechanisms of reperfusion injury. Am J Med Sci 1994;307:284-92. [Crossref] [PubMed]
- Nakao A, Sugimoto R, Billiar TR, et al. Therapeutic antioxidant medical gas. J Clin Biochem Nutr 2009;44:1-13. [Crossref] [PubMed]
- Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007;13:688-94. [Crossref] [PubMed]
- Xiang M, Fan J, Fan J. Association of Toll-like receptor signaling and reactive oxygen species: a potential therapeutic target for posttrauma acute lung injury. Mediators Inflamm 2010;2010. [Crossref] [PubMed]
- Larsson A, Guerin C. Monitoring of lung function in acute respiratory distress syndrome. Ann Transl Med 2017;5:284. [Crossref] [PubMed]
- Baxter J, Cranfield KR, Clark G, et al. Do lactate levels in the emergency department predict outcome in adult trauma patients? A systematic review. J Trauma Acute Care Surg 2016;81:555-66. [Crossref] [PubMed]
- Haegens A, Vernooy JH, Heeringa P, et al. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur Respir J 2008;31:252-60. [Crossref] [PubMed]
- Sato H, Kasai K, Tanaka T, et al. Role of tumor necrosis factor-alpha and interleukin-1beta on lung dysfunction following hemorrhagic shock in rats. Med Sci Monit 2008;14:BR79-87. [PubMed]
- Kotteas EA, Boulas P, Gkiozos I, et al. The intercellular cell adhesion molecule-1 (icam-1) in lung cancer: implications for disease progression and prognosis. Anticancer Res 2014;34:4665-72. [PubMed]
- Singh SR, Sutcliffe A, Kaur D, et al. CCL2 release by airway smooth muscle is increased in asthma and promotes fibrocyte migration. Allergy 2014;69:1189-97. [Crossref] [PubMed]
- Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 2015;26:475-87. [Crossref] [PubMed]
- Goldman JL, Sammani S, Kempf C, et al. Pleiotropic effects of interleukin-6 in a "two-hit" murine model of acute respiratory distress syndrome. Pulm Circ 2014;4:280-8. [Crossref] [PubMed]
- D'Alessio FR, Tsushima K, Aggarwal NR, et al. Resolution of experimental lung injury by monocyte-derived inducible nitric oxide synthase. J Immunol 2012;189:2234-45. [Crossref] [PubMed]