Indications and outcomes in adult lung transplantation
Introduction
Lung transplantation (LTx) is the only therapeutic option for end-stage parenchymal lung diseases or pulmonary vascular disorders. In 1963, Hardy et al. (1) performed the first lung transplant in a 58-year-old male patient who died of nephrotoxicity. Since then, significant advancements have occurred regarding organ preservation, extracorporeal support of both donor organs and recipients, surgical techniques, immunosuppressive therapeutic agents, and allograft surveillance, along with the advent of multidisciplinary, collaborative medical and surgical teams to provide care to patients after LTx. The purpose of this brief review is to review indications for LTx in adult patients and to present clinical outcomes.
Recent trends in lung transplant numbers
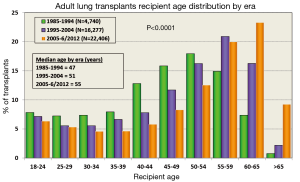
The International Society for Heart and Lung Transplantation (ISHLT) Registry provides detailed annual information on patients who have undergone LTx. The most recent report in 2013 summarized data from 43,428 adult lung and 3,703 adult heart-lung transplant recipients and their donors through June 30, 2012 (2). The number of lung transplants has continued to rise, especially over the last 5 years (Figure 1); however, this increase in demand for organs has coincided with a reduction in number of available donor lungs (2,3). Coinciding with the increase in total lung transplants, patients who are older than 65 years undergoing LTx are on the rise (Figure 1) (2,3). Similarly, the age of donor lung allografts is on the rise (4).
Indications for lung transplantation (LTx) in adults
The decision to perform LTx is a complex treatment that carries considerable surgical risks. Table 1 shows the indications for lung transplants in adults performed between January 1995 and June 2012, while Figure 2 provides the major indications by year from 1990 to 2011 (2). Revision of international guidelines for lung transplant candidates was last published in 2006 by Orens et al. (5) with a revised update being published soon, which will include pediatric recommendations for the first time.
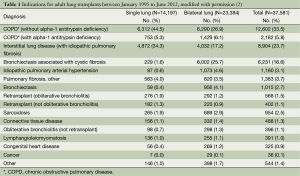
Full table
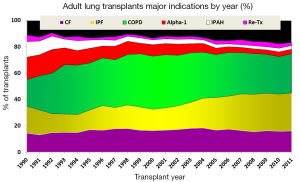
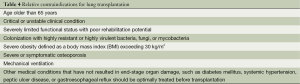
Table 2 lists the major disease categories that should be considered for LTx. Patients with these pulmonary disorders should be referred for consideration for LTx at any point if these characteristics exist or if the patient or primary healthcare provider has further questions regarding the potential benefit of LTx. Tables 3,4 outlines both absolute and relative contraindications for LTx as recently recommended. In short, LTx should not be considered in a patient with a florid infection, recent malignant tumor, continued addictive behavior, or lacks reliable social support. Infectious issues are different in cystic fibrosis with controversy continuing with most centers generally not offering transplant in patients colonized with Burkholderia cenocepacia and extreme caution used in offering transplant in the presence of Mycobacterium abscessus. Relative contraindications are determined by the individual centers with updated recommendations under development to be soon available.

Full table
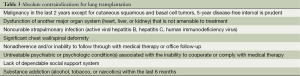
Full table
Full table
Clinical outcomes
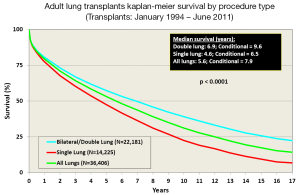
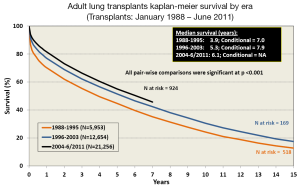
Survival after LTx in adult patients has slowly improved over the last 30 years (2). One contributing factor is the increasing number of bilateral lung transplants being performed, especially in the younger patient population (Figure 3). The improvement in survival has improved in a stepwise fashion as outlined in Figure 4.
Innovations
Hardy et al. were clearly innovative in 1963 when they performed the first lung transplant. Novel discoveries continue to influence the outcomes of patients with advanced lung disease regarding LTx. The use of extracorporeal support has made an immediate impact as it is commonplace for patients to be bridged to LTx with extracorporeal membrane oxygenation (ECMO) (6-16), but ECMO remains to be a relative contraindication in the current published guidelines, thus the need for an update. The use of ECMO as a means to bridge was recently reported with similar outcomes as lung retransplantation (6). A major innovation with the advent of normothermic ex vivo lung perfusion by the group at the University of Toronto has resulted in the successful transplantation of donor lungs that would have been previously discarded (17,18). This technology uses extracorporeal means to support donor organs. More recently, induction immunosuppression was shown to have a significantly positive effect on survival (19). Discoveries continue to include modifications of currently available treatments as best practice still continues to evolve in LTx.
Conclusions
Based on the recent advancements, the future is very bright in the care of patients with advanced lung disease who require LTx. Despite recent novel discoveries and innovations, further work is needed to improve and enhance not only the current technologies and treatments, but how we use them and in what clinical situation. Multi-center studies are badly needed in order to even further improve outcomes in LTx.
Acknowledgements
No funding was required to complete this work which was completed at The Ohio State University and Nationwide Children’s Hospital.
Disclosure: The authors declare no conflict of interest.
References
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065-74. [PubMed]
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [PubMed]
- Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. OPTN/SRTR 2010 Annual Data Report, 2011 [May 17, 2014]. Available online: http://srtr.transplant.hrsa.gov/annual_reports/2011/default.aspx
- Abecassis M, Bridges ND, Clancy CJ, et al. Solid-organ transplantation in older adults: current status and future research. Am J Transplant 2012;12:2608-22. [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [PubMed]
- Hayes D Jr, Higgins RS, Kilic A, et al. Extracorporeal Membrane Oxygenation and Retransplantation in Lung Transplantation: An Analysis of the UNOS Registry. Lung 2014;192:571-6. [PubMed]
- Fischer S, Simon AR, Welte T, et al. Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg 2006;131:719-23. [PubMed]
- Ricci D, Boffini M, Del Sorbo L, et al. The use of CO2 removal devices in patients awaiting lung transplantation: an initial experience. Transplant Proc 2010;42:1255-8. [PubMed]
- Haneya A, Philipp A, Mueller T, et al. Extracorporeal circulatory systems as a bridge to lung transplantation at remote transplant centers. Ann Thorac Surg 2011;91:250-5. [PubMed]
- Hämmäinen P, Schersten H, Lemström K, et al. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant 2011;30:103-7. [PubMed]
- Bermudez CA, Rocha RV, Zaldonis D, et al. Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg 2011;92:1226-31; discussion 1231-2. [PubMed]
- Hayes D Jr, Kukreja J, Tobias JD, et al. Ambulatory venovenous extracorporeal respiratory support as a bridge for cystic fibrosis patients to emergent lung transplantation. J Cyst Fibros 2012;11:40-5. [PubMed]
- Bittner HB, Lehmann S, Rastan A, et al. Outcome of extracorporeal membrane oxygenation as a bridge to lung transplantation and graft recovery. Ann Thorac Surg 2012;94:942-9; author reply 949-50. [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [PubMed]
- Lang G, Taghavi S, Aigner C, et al. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation 2012;93:729-36. [PubMed]
- Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7; discussion 867-8. [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [PubMed]
- Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. [PubMed]
- Whitson BA, Lehman A, Wehr A, et al. To induce or not to induce: a 21st century evaluation of lung transplant immunosuppression’s effect on survival. Clin Transplant 2014;28:450-61. [PubMed]


