
Usefulness of impulse oscillometry for the assessment of bronchodilator response in elderly patients with chronic obstructive airway disease
Introduction
Chronic obstructive airway diseases, including asthma and chronic obstructive pulmonary disease (COPD), are characterized by chronic airway inflammation and airflow limitation and are one of the most common diseases in elderly individuals. The two conditions can be distinguished by differences in the age of onset, clinical manifestations, smoking history, atopic status, and comorbidities (1,2). However, this discrimination can be particularly difficult in older populations. In addition, patients frequently present features of both diseases, which is referred to as asthma-COPD overlap syndrome (ACOS) (1,3,4). Recent guidelines have proposed diagnostic criteria to distinguish asthma from COPD by clinical assessment of symptoms and demonstration of airflow limitation (1). However, spirometry performance remains challenging for many elderly patients who have comorbidities that affect the test procedure and in cases where patient cooperation is lacking. Therefore, a more readily available test for bronchodilator response (BDR) is crucial for patient assessment.
Impulse oscillometry (IOS) measures airway resistance and reactance during tidal breathing. While conventional spirometry requires a forced expiratory maneuver, IOS is an effort-independent and patient-friendly modality for evaluating lung function and peripheral airway dysfunction (5). Recent studies have shown that IOS could be useful for diagnosing asthma and assessing asthma control, especially in children (6,7). Although IOS is being used increasingly in various asthma and COPD studies, its clinical utility remains unclear. To date, few investigations have used IOS parameters to evaluate airway reversibility in asthma and COPD (8,9).
This study aimed to evaluate whether IOS could demonstrate a BDR and play a role as an alternative to spirometry in elderly patients with chronic obstructive airway disease, such as asthma and COPD. In addition, we examined differences in IOS parameters between patients with asthma and COPD. Furthermore, the sensitivity and specificity of IOS measurements for identification of the BDR were computed.
Methods
Patients
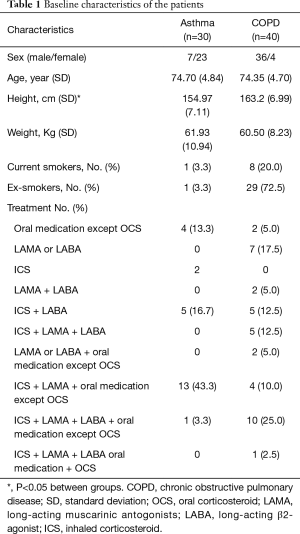
The study enrolled 70 patients (>65 years of age) with asthma (n=30) or COPD (n=40) from the Haeundae Paik Hospital’s outpatient clinics at the Inje University in Busan, Korea between June 2011 and October 2014. The study was approved by the hospital’s medical ethics committee (approval number: 2012-090), and informed consent was obtained from all the enrolled patients. Asthma diagnosis was based on a clinical history of variable respiratory symptoms and pulmonary function according to the Global Initiative for Asthma (GINA) guidelines (1). COPD was diagnosed based on a clinical history of progressive, exertional dyspnea and pulmonary function characterized by not fully reversible airflow obstruction, which was defined as forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70% after bronchodilator administration (2). Patients with COPD had a smoking history of more than 20 pack-years. One pack-year was defined as twenty cigarettes smoked every day for one year. Patients who were not willing to enroll in the study and those with moderate-to-severe bronchiectasis, tuberculosis-destroyed lung, interstitial lung disease, active respiratory infectious disease, severe mental illness, moderate-to-severe heart failure, malignancy, and other severe systemic diseases were also excluded.
Study design
This was a single-center prospective study. Before inclusion, physicians reviewed the medical history and disease control status of each patient. In patients without a history of recent exacerbation over at least 4 weeks, baseline spirometry and IOS measurements were recorded. The patients allowed maintained use of their prescribed medication without any changes at least before 4 weeks. Albuterol was administrated as two puffs of 100 µg through a pressurized metered dose inhaler. Spirometry and IOS were repeated 15 minutes after albuterol administration. IOS was always performed before spirometry to avoid the influence of the forced maneuver.
Spirometry
We used the computerized spirometers, Vmax Encore 22D and 29C (SensorMedics Corp., Yorba Linda, California, USA), for all measurements. Spirometry was performed according to American Thoracic Society (ATS) guidelines (10). FVC, FEV1, FEV1/FVC, and forced expiratory flow at 25–75% (FEF25–75) were measured. Predicted spirometry values were calculated in accordance with Choi’s equation, which has been validated for the Korean population (11). The best of at least three technically acceptable results was selected.
IOS
IOS was performed using the MasterScreen IOS system (Cardinal Health Germany, 234 GmbH, Hoechberg, Germany) following a standardized protocol based on the manufacturer’s instructions. Each patient was seated upright, wore a nose clip, and pressed on their cheeks with their hands to prevent an upper airway shunt. To avoid air leakage, patients sealed their lips tightly around the mouthpiece. While the impulse produced by the speaker is moving with patient’s breathing, a pressure and flow transducer measured inspiratory and expiratory flow and pressure changes in the respiratory system. Mean respiratory resistance values were calculated over a period of 30 seconds in a frequency range of 5 to 35 Hz. IOS parameters such as resistance at 5 Hz (R5), resistance at 20 Hz (R20), frequency dependence of resistance calculated as the difference between resistance at 5 and 20 Hz (R5–20), reactance at 5 Hz (X5), resonant frequency (Fres), and area of reactance (AX) were recorded. R5 and R20 represent total airway resistance and resistance of the central, large airway, respectively. In central, large airway obstruction, the resistance increases at all frequencies. Conversely, in small airway obstruction, the resistance at lower frequencies increases but is unchanged at higher frequencies that do not reach the small airways. Reactance at low frequencies, such as X5, can provide information about the distal airway. Fres represents the degree of airway obstruction and is elevated in both restrictive and obstructive pulmonary disease. AX is another common parameter, and represents the total reactance at all frequencies between 5 Hz and Fres (5,12,13). In the present study, we rejected IOS measurements with coherence values <0.6 between 5 and 15 Hz, or <0.8 for frequencies >20 Hz. The best of three acceptable attempts with the lowest respiratory resistances was chosen for the final data analysis. During this study, one technician obtained all IOS measurements.
Data and statistical analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC, USA). Data were assessed for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests before further analysis. Significance was defined as a P<0.05. Coefficients of correlation and regression analysis were used to identify the correlations between percentage changes in spirometry measurements and IOS parameters after bronchodilator administration. A t-test was used to compare means of variables of interest between the bronchodilator positive and control groups. Subgroup analyses were performed to identify differences associated with the BDR, defined as an FEV1 change >12% of the predicted value and an increase in volume >200 mL (14) in both bronchodilator positive and negative groups by using the Mann-Whitney test. In addition, subgroup analysis was performed to identify the differences after reclassifying asthma and ACOS according to the GINA 2014 (1,3,4) and the COPD group using the Mann-Whitney test. The receiver operating characteristic curve (ROC) method was used to evaluate the utility of IOS parameters in identifying BDR. ROC area under the curve (AUC) values with estimated standard error and optimal IOS cutoff values based on maximizing the sum of sensitivity and specificity were calculated for each of the IOS values. We analyzed the AUC as the value of change for each of the IOS values before and after bronchodilator use.
Results
Baseline patient characteristics
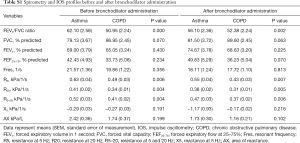
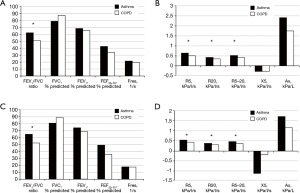
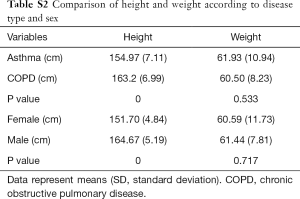
Baseline patient characteristics are presented in Table 1. The mean patient age in the asthma and COPD groups was 74.7±4.8 and 74.4±4.7 years, respectively (P=0.761). The mean height of the asthma group was significantly lower than that of the COPD group. This was due to a sex ratio imbalance between groups. There was no significant difference in weight between groups (Table S1). There were no intergroup differences in spirometry parameters, such as FVC, FEV1, and FEF25–75, before or after bronchodilator administration. However, IOS indices representing airway resistance, such as R5, R20, and R5–20, were significantly higher in the asthma group compared with those in the COPD group both before and after bronchodilator administration. There were no significant intergroup differences in Fres, X5, and AX (Figure 1, Table S2).
Full table
Full table
Full table
Correlation analysis between percentage changes in IOS and spirometry parameters after bronchodilator administration
Correlations between percentage changes in IOS and spirometry parameters after bronchodilator administration were analyzed in all patients. The percentage change in FEV1 showed significant correlation with the changes in all IOS parameters. In particular, the correlations between percentage changes in FEV1 and those in R5 and R5–20 were relatively strong with r values of −0.671 (P<0.001) and −0.640 (P<0.001), respectively. In addition, significant correlations were also observed between the percentage change in FEF25–75 and the percentage changes in IOS parameters (Figure 2, Table S3).
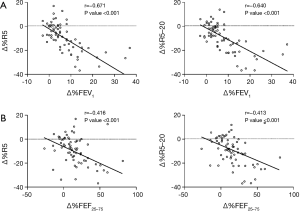
Full table
Discrimination between asthma and COPD using percentage changes in IOS parameters after bronchodilator administration
We attempted to discriminate between the asthma and COPD groups on the basis of the percentage changes in IOS parameters before and after bronchodilator administration. There were no significant intergroup differences in the percentage changes in R5 and R5–20, which showed the strongest correlation with spirometry in the whole-group analysis (Figure 3). In addition, there were no significant differences between groups in the other IOS parameters, such as Fres, R20, X5, and Ax (Table S4). However, when we reclassified ACOS under the asthma group, Fres, R5, and R5–20 showed significant differences between the asthma + ACOS group and COPD group (Table 2).


Full table
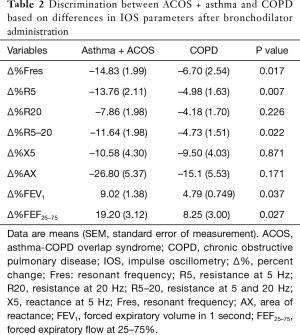
Full table
Proposed cutoff values of IOS parameters for identifying BDR
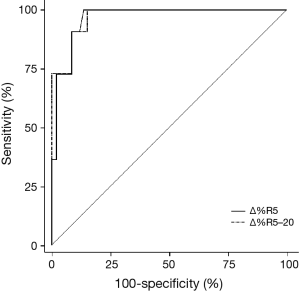
Table 3 shows the numerical analysis of the ROC curve, including the AUC, sensitivity, specificity, and optimal cutoff values for each IOS parameter presented as percentage changes after bronchodilator administration. The sensitivity, specificity, and cutoff values from the ROC curves were compared with respect to BDR, which was classified on based on FEV1 values. Among the IOS parameters that showed statistical significance for BDR, the best cutoff point was −15.4%, which was the percentage change in R5–20 (sensitivity, 100%; specificity, 84.75%). When the discriminative properties of the percentage changes in IOS parameters for identifying a BDR were shown using ROC curves, the best profile for detecting BDR was obtained with R5–20, which had the highest AUC (0.971), followed by R5 (0.967) (Figure 4).
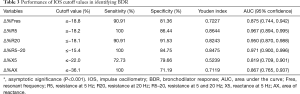
Full table
Discussion
In this study, we identified BDR in asthma and COPD patients using IOS, which is consistent with the results of previous studies (9,15-17). There is an association between parameters such as respiratory impedance, reactance, and resistance using the forced oscillation technique (FOT) and the FEV1, FVC, lung volume, and respiratory symptoms before and after use of Short-acting β agonist (SABA), long-acting β2-agonist (LABA), and inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) in patients with COPD (18-21). Interestingly, spirometry, which is the standard method for measurement of pulmonary function, is unlikely to reflect the pathophysiology of small airway disease; therefore, there are limitations in detecting changes in small airways and airway trapping in patients with asthma and COPD. Importantly, we found that IOS differentiated small airway obstruction from large airway obstruction and was more sensitive than spirometry for peripheral airway disease. In line with this, the 2017 revision Global Initiative for Chronic Obstructive Lung Disease (GOLD) introduced a refinement of the clinical guidance system by separating spirometric evaluations. Spirometry remains a key tool in the diagnosis of COPD; however, it is excluded from pharmacotherapy recommendations. This revised assessment tool acknowledges the limitation of FEV, which can affect some therapeutic decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks (2). IOS may be another option to replace spirometry because it detects the early pathophysiological changes in COPD that were not found on spirometry (21).
Recently, an oscillometry technique that can measure actual airway resistance and impedance has been described with extensive studies in pediatric patients (7,15,22-28). To date, many studies on the BDR of COPD patients have used the FOT method. The IOS is a variant of the FOT, which include some additional features and benefits. In FOT, sound waves of different frequencies are transmitted sequentially. This provides good temporal resolution for the measurement of respiratory resistance. In IOS, a pulse, which can be mathematically decomposed into different frequencies, is transmitted. This pulse, which contains all frequencies from 5–30 Hz, is transmitted into the lung. IOS has some advantages compared with FOT. IOS can calculate the impedance at every frequency from 5–30 Hz while FOT calculates at specific sine wave frequencies. In addition, the use of IOS decreases test duration. IOS shows improved signal to noise ratio; therefore, it is beneficial for detecting regional abnormalities. However, there are some disadvantages. IOS can be more forceful for the patient when compared with the gentler plain sinusoidal waves of FOT. Finally, it may change the lung mechanics slightly (13,29).
In clinical practice, the greatest advantage of IOS is that it requires no effort from the patient. The effort-independent nature of this method for evaluating lung function during normal tidal breathing is a notable characteristic of IOS, making it easy to use in children and patients with physical and cognitive limitations (22,30). IOS is useful for evaluating airway obstruction and bronchodilator responsiveness in pediatric asthma patients. Recent studies have also reported standard values and cutoff ranges for BDR in children (6,7,9,23-27,31). In addition, several studies in adult patients with asthma and COPD (32-34) have reported that IOS parameters correlate better with asthma control in adults than spirometry indices (35). One study has suggested that IOS should be the preferred method to measure bronchodilation in COPD (36). However, the clinical implications of using the IOS index in adult patients remains under discussion. Furthermore, several studies have stated that no acceptable reference values are available for adults (30). Nevertheless, IOS indices have been suggested to be good markers not only for the diagnosis of asthma and COPD but also for the evaluation of disease control in elderly patients who experience difficulties while performing spirometry and the bronchial provocation test (9,27,28).
In our study, the percentage changes in IOS parameters after bronchodilator administration significantly correlated with the percentage changes in FEV1 in elderly patients with asthma and COPD. Confirming BDR is important for patient assessment; therefore, IOS may be useful for finding treatable components. Our results also showed a correlation between FEF25–75 and changes in IOS parameters. This may be due to a reduction in airway trapping via bronchodilator treatment leads to a reduction in resistance value, leading to increased small airway recruitment and symptom improvements. Therefore, IOS may be useful for evaluating small airway disease in elderly patients with asthma and COPD.
It was difficult to distinguish between the asthma and COPD groups using either spirometry or IOS after bronchodilator administration in the present study. These were primarily because our patients continued to receive medication for asthma or COPD and were in a stable condition. Therefore, the post-bronchodilator differences were insufficient to discriminate between the two groups. Furthermore, some IOS parameters showed different results than we expected. Patients with small airway disease are predicted to have higher Fres and AX, and lower X5. However, there were no differences between the asthma and COPD groups in this study. The first possible explanation is that the longer the disease duration of asthma, the greater occurrence of more structural changes in the airway, such as airway remodeling, can occur, which can also affect the IOS values. Second, the mean height of the asthma patients was significantly lower than the COPD group, due to an imbalance in sex ratio between groups. Decreased height can affect the IOS parameters. As a result, Fres and resistance were increased and X5 was decreased (13,29). Nevertheless, when we classified asthma and ACOS in the same group, changes to some IOS parameters (R5, R10, R5–20, and Fres) after bronchodilator administration showed significant differences. The ACOS should be separated from the COPD group to differentiate between asthma and COPD; however, confirming BDR is more clinically useful in the evaluation of treatable characteristics. In additional studies, we plan to IOS in a clinical setting to distinguish asthma, COPD, and ACOS. We also attempted to identify the cutoff values to detect BDR because of the importance of these values in developing a treatment strategy for chronic airway obstructive disease. We found that all IOS parameters had high AUCs.
Interestingly, R5 and R5–20 values were higher in the asthma group prior before and after bronchodilator administration in our study. This was inconsistent with the findings of a previous study, in which the asthma group was differentiated from the COPD group by analysis of the differences between inspiratory and expiratory IOS parameters, although the authors of that study failed to assess discrimination by whole-breath IOS analysis (37). Previous studies have shown that IOS can measure the small airway pathophysiology not measured by spirometry (38,39). Considering the populations in these studies, small airway obstruction may have been more severe in our asthma group than in previous studies. Since the results of a study in children showed that IOS parameters can predict asthma control, it is possible that asthma control in the patients in our asthma group was insufficient (7). Larger studies are needed to elucidate differences in IOS indices between asthma and COPD and to evaluate whether increased small airway obstruction improves after intensive treatment.
There were several limitations in our study. First, we could not enroll a normal control group. However, previous studies have shown significant differences in IOS values between healthy controls and patients with asthma or COPD (9,27,28,37). Second, we have proposed a cutoff value that can define BDR through changes in IOS parameters. The sample size in our study was too small to apply our optimal cutoff values for BDR in a clinical setting; therefore, we plan to conduct a larger study to obtain more reliable results. Third, there was a difference in the mean height between the asthma and COPD groups due to differences in the sex ratio between groups. The lower mean height in the asthma group may result in increased resistance and Fres and decreased reactance.
Conclusions
Our results show that IOS is a patient-friendly complement to spirometry, which may help elderly patients with asthma or COPD who have difficulty performing spirometry. We found that IOS showed a good correlation with the index of airway obstruction in spirometry and also detected BDR well. Notably, it may be essential to use IOS more than spirometry, which has many limitations with regard to the patient’s condition and effort.
Acknowledgements
Funding: This work was supported by a grant from Research year of Inje University in 2017 (grant number 20170101).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Haeundae Paik Hospital’s medical ethics committee (IRB number: 2012-090). Informed consent was obtained from all the enrolled patients.
References
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Revised 2014. Accessed on 1 Feburary 2015. Available online: http://www.ginasthma.org
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, management, and Prevention of Chronic Obstructive Pulmonary Disaese. Updated 2014. Accessed on 1 Feburary 2015. Available online: http://www.goldcopd.org
- Soler-Cataluña JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol 2012;48:331-7. [Crossref] [PubMed]
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009;64:728-35. [Crossref] [PubMed]
- Bickel S, Popler J, Lesnick B, et al. Impulse oscillometry: interpretation and practical applications. Chest 2014;146:841-7. [Crossref] [PubMed]
- Nève V, Edmé JL, Devos P, et al. Spirometry in 3-5-year-old children with asthma. Pediatr Pulmonol 2006;41:735-43. [Crossref] [PubMed]
- Shi Y, Aledia AS, Galant SP, et al. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol 2013;131:718-23. [Crossref] [PubMed]
- Olaguíbel JM, Alvarez-Puebla MJ, Anda M, et al. Comparative analysis of the bronchodilator response measured by impulse oscillometry (IOS), spirometry and body plethysmography in asthmatic children. J Investig Allergol Clin Immunol 2005;15:102-6. [PubMed]
- Nair A, Ward J, Lipworth BJ. Comparison of bronchodilator response in patients with asthma and healthy subjects using spirometry and oscillometry. Ann Allergy Asthma Immunol 2011;107:317-22. [Crossref] [PubMed]
- Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107-36. [Crossref] [PubMed]
- Choi JK, Paek D, Lee JO. Normal predictive values of Spirometry in Korean population. Tuberc Respir Dis 2005;58:230-42. [Crossref]
- Dubois AB, Brody AW, Lewis DH, et al. Oscillation mechanics of lungs and chest in man. J Appl Physiol 1956;8:587-94. [Crossref] [PubMed]
- Desiraju K, Agrawal A. Impulse oscillometry: The state-of-art for lung function testing. Lung India 2016;33:410-6. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2- to 5-year-old asthmatic children as measured by impulse oscillometry. J Asthma 2002;39:531-6. [Crossref] [PubMed]
- Sheen YH, Jee HM, Ha EK, et al. Impulse oscillometry and spirometry exhibit different features of lung function in bronchodilation. J Asthma 2018;55:1343-51. [Crossref] [PubMed]
- Short PM, Williamson PA, Lipworth BJ. Sensitivity of impulse oscillometry and spirometry in beta-blocker induced bronchoconstriction and beta-agonist bronchodilatation in asthma. Ann Allergy Asthma Immunol 2012;109:412-5. [Crossref] [PubMed]
- Milne S, Hammans C, Watson S, et al. Bronchodilator responses in respiratory impedance, hyperinflation and gas trapping in COPD. COPD 2018;15:341-9. [Crossref] [PubMed]
- Ito S, Uchida A, Isobe Y, et al. Responsiveness to bronchodilator procaterol in COPD as assessed by forced oscillation technique. Respir Physiol Neurobiol 2017;240:41-7. [Crossref] [PubMed]
- da Costa GM, Faria AC, Di Mango AM, et al. Respiratory impedance and response to salbutamol in healthy individuals and patients with COPD. Respiration 2014;88:101-11. [Crossref] [PubMed]
- Timmins SC, Diba C, Schoeffel RE, et al. Changes in oscillatory impedance and nitrogen washout with combination fluticasone/salmeterol therapy in COPD. Respir Med 2014;108:344-50. [Crossref] [PubMed]
- Frei J, Jutla J, Kramer G, et al. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest 2005;128:1266-73. [Crossref] [PubMed]
- Shin YH, Yoon JW, Choi SH, et al. Use of impulse oscillometry system in assessment of asthma severity for preschool children. J Asthma 2013;50:198-203. [Crossref] [PubMed]
- Marotta A, Klinnert MD, Price MR, et al. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol 2003;112:317-22. [Crossref] [PubMed]
- Song TW, Kim KW, Kim ES, et al. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol 2008;19:763-8. [Crossref] [PubMed]
- Bailly C, Crenesse D, Albertini M. Evaluation of impulse oscillometry during bronchial challenge testing in children. Pediatr Pulmonol 2011;46:1209-14. [Crossref] [PubMed]
- Nielsen KG, Bisgaard H. Discriminative capacity of bronchodilator response measured with three different lung function techniques in asthmatic and healthy children aged 2 to 5 years. Am J Respir Crit Care Med 2001;164:554-9. [Crossref] [PubMed]
- Shin YH, Jang SJ, Yoon JW, et al. Oscillometric and spirometric bronchodilator response in preschool children with and without asthma. Can Respir J 2012;19:273-7. [Crossref] [PubMed]
- Brashier B, Salvi S. Measuring lung function using sound waves: role of the forced oscillation technique and impulse oscillometry system. Breathe (Sheff) 2015;11:57-65. [Crossref] [PubMed]
- Schulz H, Flexeder C, Behr J, et al. Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One 2013;8:e63366. [Crossref] [PubMed]
- Komarow HD, Skinner J, Young M, et al. A study of the use of impulse oscillometry in the evaluation of children with asthma: analysis of lung parameters, order effect, and utility compared with spirometry. Pediatr Pulmonol 2012;47:18-26. [Crossref] [PubMed]
- Gong SG, Yang WL, Zheng W, et al. Evaluation of respiratory impedance in patients with chronic obstructive pulmonary disease by an impulse oscillation system. Mol Med Rep 2014;10:2694-700. [Crossref] [PubMed]
- Mineshita M, Shikama Y, Nakajima H, et al. The application of impulse oscillation system for the evaluation of treatment effects in patients with COPD. Respir Physiol Neurobiol 2014;202:1-5. [Crossref] [PubMed]
- Piorunek T, Kostrzewska M, Cofta S, et al. Impulse oscillometry in the diagnosis of airway resistance in chronic obstructive pulmonary disease. Adv Exp Med Biol 2015;838:47-52. [Crossref] [PubMed]
- Takeda T, Oga T, Niimi A, et al. Relationship between small airway function and health status, dyspnea and disease control in asthma. Respiration 2010;80:120-6. [Crossref] [PubMed]
- Borrill ZL, Houghton CM, Woodcock AA, et al. Measuring bronchodilation in COPD clinical trials. Br J Clin Pharmacol 2005;59:379-84. [Crossref] [PubMed]
- Paredi P, Goldman M, Alamen A, et al. Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax 2010;65:263-7. [Crossref] [PubMed]
- Pisi R, Tzani P, Aiello M, et al. Small airway dysfunction by impulse oscillometry in asthmatic patients with normal forced expiratory volume in the 1st second values. Allergy Asthma Proc 2013;34:e14-20. [Crossref] [PubMed]
- Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007;132:1275-82. [Crossref] [PubMed]


