Heart-lung transplantation: pediatric indications and outcomes
Indications for pediatric heart lung transplantation (HLT)
The first HLT was performed in 1981 for a patient with primary pulmonary hypertension (PPHN) (1). The second HLT was performed for Eisenmenger’s syndrome with unrepaired congenital heart disease. Indications for HLT remain similar today (2).
Combined heart double lung transplant is typically offered in cases with end-stage dysfunction of both the heart and the lungs (3). Multi-organ transplantation comes with increased risk to the patient and so heart transplantation (HT) or double lung transplantation (DLT) is considered instead of HLT and preferred when only one organ is affected to the extent of causing end-stage disease. HLT was performed in infants in the 1980s and 1990s for technical reasons instead of isolated heart or lung transplants, but this practice has largely disappeared as technical issues have been overcome with advances in surgery and greater expertise (4). In cases where patients have end-stage lung disease associated with or causing cardiac dysfunction, congenital heart disease with pulmonary hypertension, or congenital heart disease associated with pulmonary artery/vein abnormalities, HLT may be indicated (5,6). HLT may also be considered for retransplantation following either HT or lung transplantation. In the case of pulmonary hypertension, if cardiac function is preserved, DLT alone is indicated. In the case of pulmonary hypertension with severe right heart failure or left heart failure, HLT would then be indicated. It should be stressed that each case needs to be considered carefully and each organ closely scrutinized to determine the need for transplantation. The ability of the right heart to recover function can be difficult to predict and needs to have careful consideration when deciding the best option for the patient (HLT or DLT) (7). Care must be individualized as there are cases in which the stress of transplanting the “end-stage” organ would further compromise the function of the other and so it would be in the patient’s best interest to perform HLT.
Evaluation for HLT typically occurs when a patient has an underlying disease compromising cardiac and pulmonary function and has a predicted survival of less than two years due to that underlying disease. A predicted survival of greater than two years would suggest that a patient would not derive a survival benefit from HLT and should not be listed for HLT given current outcomes (3). HLT is most indicated when patients have a survival expectancy of a few months to a year. Survival expectancy of less than a few months risks death while awaiting transplantation due to current wait times on the HLT list. Requiring inotropic support, mechanical ventilation, and/or mechanical circulatory support prior to transplant also lowers survival and should increase the urgency and priority for HLT. Candidates who are ambulatory and have adequate nutrition prior to transplantation derive better outcomes and so it is best to evaluate and list for HLT before severe, life-threatening complications arise. Body mass index and ideal body weight are parameters that can help determine whether HLT outcomes might be affected by poor nutrition (8). Finally, HLT should be considered if quality of life (QOL) is significantly impacted to the point that children are not able to participate in school or if they are dependent upon cardio-respiratory support that impinges upon QOL.
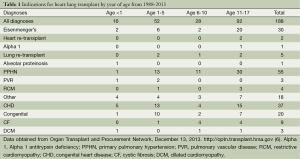
The three most common reasons in the United States for which patients have received a HLT since 1988 are PPHN (29%), congenital heart disease (CHD) (20%), and Eisenmenger’s syndrome (16%); as shown in Table 1 (9). By the ISHLT data, worldwide the most common reasons for which patients have received a HLT since 1986 are cystic fibrosis (28%), pulmonary hypertension (24%), congenital disease (22%), and Eisenmenger’s syndrome (12%) (3). Other indications for HLT include heart re-transplantation, lung re-transplantation, alpha-1-antitrypsin deficiency, alveolar proteinosis, pulmonary vascular disease, restrictive cardiomyopathy, dilated cardiomyopathy, chronic obstructive pulmonary disease, and restrictive pulmonary disease. From 2000 to 2012, ISHLT data for diagnosis show more patients receive a HLT with an indication of cystic fibrosis in Europe than in North America. More patients receive a HLT with an indication of congenital heart disease in North America than in Europe (3).
Full table
Indications for isolated HT include lethal congenital heart disease in the newborn; end-stage congenital heart disease in the older child not amenable to palliative or corrective cardiac surgery; end stage cardiomyopathy; recurrent life threatening arrhythmias not controlled by medications, implanted defibrillator, or ablation; failure to wean from mechanical circulatory support; heart retransplantation; or other cardiac disease with a predicted survival less than 2 years (10). Since outcomes for DLT are poor in comparison to HT, HLT should only be considered if lung function is severely compromised and progressive in nature. The most common indications for isolated DLT are cystic fibrosis and pulmonary hypertension (3). Since cardiac function can be severely compromised by end-stage lung disease, HLT may need to be considered for those with severe primary lung disease that has affected cardiac function to the point that cardiac dysfunction is considered to be progressive and irreversible (7).
Contraindications for HLT include extra-cardiac disease such as severe end-organ disease (such as renal or hepatic disease), active/recent malignancy, HIV infection, or other infection that is active or resistant to treatment (10). Because outcomes for HLT are not great and surveillance is rigorous, relative or absolute contraindications to HLT may also be psychosocial issues such as severe depression, psychiatric disease or poor adherence to medical regimens (11). Previous thoracic surgery can complicate the technical aspects of the transplantation and so this may also need to be taken into consideration. Transplant from mechanical ventilation or mechanical support such as venous arterial (VA)/venous venous (VV) extracorporal membranous oxygenation (ECMO) is high risk. Our center will consider transplant from mechanical ventilation or VV ECMO. With complex congenital heart disease, significant aortopulmonary collaterals may develop with are a relative contraindication. Allosensitization increases the risk of rejection and graft failure post transplant and is a relative contraindication. Contraindications to isolated heart or DLT are similar.
Indications by year of age for HLT are summarized in Table 1 (9). Congenital heart disease is more common as an indication in younger patients. PPHN is more common in older children. Eisenmenger’s syndrome is most common in older children. A review of data from the Organ Procurement and Transplantation Network (OPTN) of the indications for heart lung transplant from 1/1/1988 through 7/31/2013 show that the number of HLT performed over the last 2 decades has remained stable. Since 1988, 188 HLT have been performed in individuals under the age of 18 at 33 centers in the United States. Of those, 48% were male, 47% blood group A, 41% blood group O, 77% Caucasian not of Hispanic origin. Nearly half (48.9%) of recipients were ages 11-17. In the same time period, 949 HLT were performed in adults 18 years of age and older. Pediatric HLT made up 16.5% of HLT performed in that era (9).
When the data from the OPTN is stratified by age groups, 16 (8.5%) were less than 1 year of age, 52 (27.7%) were ages 1-5, 28 (14.89%) were ages 6-10, and 92 (48.9%) of HLTs performed were in children ages 11-17 (9). In the youngest age group (less than 1 year), the three most common indications were congenital heart disease (31.25%), other (25%), and Eisenmenger’s syndrome (12.5%). Only one recipient had a diagnosis of PPHN. One recipient had a diagnosis of pulmonary vascular disease. No HLTs have been reported in recipients less than 1 year of age since 2007. Children in the middle age groups (1-5 and 6-10) received HLT most commonly for PPHN and congenital heart disease. Children in the oldest age group (11-17) were also mostly transplanted for PPHN and congenital heart disease, but this is the age group in which Eisenmenger’s syndrome was more common than the others. For data from ISHLT from 1982 to 2012 stratified by age groups, 21 (3%) were less than 1 year of age, 106 (15.6%) were ages 1-5, 127 (18.7%) were ages 6-10, and 425 (62.6%) of HLT performed were in children ages 11-17 (3).
When the OPTN data is stratified by era (Table 2), one notices that the overall number of HLTs has decreased in the most recent era [2008-2013] (9). Further dissection of the OPTN data would show that Eisenmenger’s as an indication for HLT has decreased. Thirty recipients received HLT for Eisenmenger’s syndrome up until 2002 and since then there have not been any HLTs performed for Eisenmenger’s syndrome. PPHN and congenital heart disease have been common indications through all eras.

Full table
ISHLT data stratified by era also shows an overall decrease in the number of HLTs in the recent era, from a peak of 60 HLT in 1989 to <10 in 2011. Age distribution by era comparing 1982-1999 to 2000-2012 shows an increase in the percentage of patients who are ages 11-17 and <1 year of age at the time of transplant, and decrease in the percentage of patients who are ages 1-5 and 6-10 at the time of transplant. There is a decrease in the number of patients transplanted for cystic fibrosis and an increase in patients transplanted for pulmonary hypertension and congenital disease (3).
In 2012, there were two HLTs performed in North America for children less than 18 (12). Both recipients were between 11-17 years of age. This is compared to 32 HLTs that were performed in adults in 2012. Even in the adults, the most common indications were PPHN (22%) and congenital heart disease (12%). To date for 2013, there have been 5 reported HLT. Two patients were age 1-5, 1 patient age 6-10, and 2 patients age 11-17. In the last 3 years, 6 centers have performed a total of 9 HLTs.
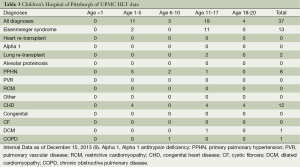
At our program, we have performed 37 HLT since 1988. Demographics with diagnoses by age are shown in Table 3 (13). Forty-nine percent were male, 35% were blood group A, 30% blood group O, 92% were Caucasian not of Hispanic origin, 12 recipients (32%) were diagnosed with PPHN, 11 (30%) with congenital heart disease, and 9 (24%) with Eisenmenger’s syndrome (13).
Full table
There have been case reports of unusual indications for HLT. Wuyts et al. described HLT in the setting of pulmonary artery dissection in patients with PPHN (14). Malignancy is typically considered a contraindication to transplantation, but cardiac tumors can be considered indications for transplantation. Talbot reported a case series of HLT for four patients with primary cardiac sarcomas involving the pulmonary artery and/or veins (15).
As discussed above, the indications for HLT have changed over the last 25 years (5). The primary indications in the USA remain PPHN, congenital heart disease, and Eisenmenger’s syndrome. Cystic fibrosis historically is a common indication but has become less prevalent. While the indications for HLT have changed over time, the overall need for HLT and HT has also changed with the improved outcomes in pulmonary hypertension and congenital cardiac surgical centers allowing for correction of the cardiac defect and possibly only needing DLT if treatment of pulmonary hypertension is not sufficient. Diagnostics and therapies for pulmonary hypertension have improved which has allowed for earlier diagnosis and treatment.
Cardiac centers are offering palliative procedures to newborns with more complicated lesions with better outcomes than in prior decades (16). For example, survival for a first-stage palliation for hypoplastic left heart syndrome, a Norwood procedure, has improved compared with 15 years ago (17). In the 1990s, many centers offered HT or HLT as primary palliation for complex congenital heart disease, including hypoplastic left heart syndrome. Furthermore, patients with simple and complex congenital heart disease are being identified at a younger age (16). Fetal echocardiography has contributed to this as well as the introduction for universal pulse oximetry screening in the newborn period. Improved detection of congenital heart disease, and more importantly, improved surgical outcomes have decreased the risk of developing Eisenmenger’s syndrome. Finally, single organ heart or double lung transplants are being performed in patients who would have received combined transplants in prior decades. This is partially as a result of better repair of congenital heart disease and ends up being helpful as there is often difficulty in obtaining heart-lung blocks
Despite all this, HLT will not become obsolete as centers are willing to perform more complex palliative operations for congenital heart disease. With advancement of medical treatment for pulmonary hypertension and surgical technique for palliation of congenital heart disease, transplant centers will need to offer HLT when medicine and surgery do not adequately treat the increasingly complex cases that are being cared for in heart and lung centers.
Outcomes
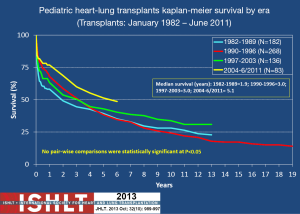
Outcomes for HLT are largely dependent upon the lung graft. Because DLT outcomes are relatively dismal in comparison to other solid organ transplants, the HLT outcomes can also be somewhat dismal. Five-year survival (Kaplan-Meier) for DLT is approximately 50% for both adults and children and remains significantly lower than survival for other solid organ transplants (3). As is shown in Figure 1, HLT survival is similar (1). A steep, early decline in survival that levels off at 1-3 months HLT reflects the impact of early events such as surgical complications, early graft failure, infection, and thromboembolism. Importantly, however, surgical outcomes have improved significantly over the last 20 years and so that steep, early decline in survival is no longer a major contributor to post-transplant mortality (also demonstrated in Figure 1). Unfortunately, the slow progressive decline that occurs after the early post-surgical period remains in both DLT and HLT. This is primarily due to chronic rejection of the lung. At our center, outcomes are similar to the national and international experience in that HT outcomes are better than those in DLT and HLT (13).
Surveillance to monitor the health of the heart-lung graft is of paramount importance when caring for children who have undergone transplantation. As previously indicated the lung graft is particularly susceptible to complications (especially rejection) and so the surveillance is mainly dictated by what is needed to assure good lung graft function. This monitoring consists of frequent evaluations. Examinations, lung function testing, evaluation of exercise capacity (6-minute walk testing), and surveillance bronchoscopy with transbronchial lung biopsies are the usual evaluations that occur to evaluate lung graft health. Specifically, since lung graft health is the limiting factor in HLT success, surveillance of the transplanted lungs with lung function, bronchoscopy, biopsy, and radiographs (often CT scanning) is important in the post-transplant period (18-23). Examinations, echocardiograms and evaluation of exercise capacity are also used to evaluate the heart graft health. Allograft rejection of the heart graft in HLT occurs less often than with HT alone (24). Therefore, rejection following HLT is most likely to occur in the lung graft. Surveillance endomyocardial biopsies are not indicated except in cases where heart rejection is suspected from cardiac studies and/or transbronchial lung biopsies are contraindicated. Monitoring for complications from immunosuppressive regimens include screening for systemic hypertension, renal insufficiency, hypercholesterolemia, diabetes, and osteoporosis (25). Finally, monitoring for conditions that are more prevalent in transplanted individuals such as post-transplant lymphoproliferative disease (PTLD) and malignancy are also of great importance (26).
The immunosuppressive regimen for any transplant that involves the lung is more intense because of the greater possibility for rejection of the lung graft. Because of this, complications that come from the immunosuppressive regimen and PTLD/malignancy need to be monitored for frequently and carefully. Because the monitoring is extensive and complications are common in the post-transplant period, many transplant experts counsel patients prior to transplant that having the procedure is like “trading one disease for another” (27). Furthermore, these outcomes have significant impact on the timing and decision to transplant as lung transplantation should occur when it is absolutely necessary in order to maximize the benefit of the transplant.
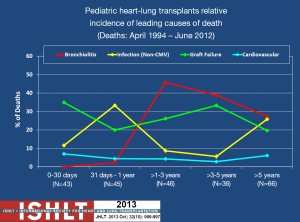
Bronchiolitis obliterans (BO) and infection have the greatest impact on long-term survival, and constant exposure of the lung to ambient air as well as aspiration of upper airway and/or refluxed gastroesophageal secretions are likely the major contributors to graft failure and death. BO is the pathologic mechanism by which chronic rejection occurs in the lung. Because of its significant prevalence and tendency to relentlessly progress, BO claims the lives of most individuals who survive the early post-operative period (28). This is the case for DLT and so this is why HLT survival primarily is dependent upon the lung graft. Figure 2 illustrates the impact that BO has in mortality following HLT and the relative non-impact that cardiovascular disease has on mortality. One can also see the impact that infection has on mortality. Again, this is due to the intensity of the immunosuppressive regimen needed to preserve lung graft health and prevent rejection.
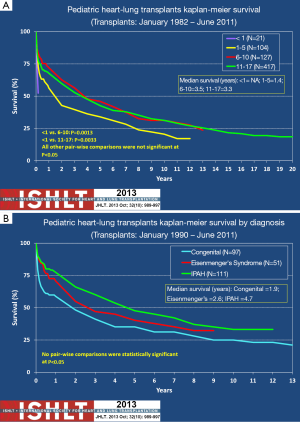
There are, however, two other factors that may affect survival of the HLT differently from DLT alone. These are recipient age and the indication for transplantation. While minor in comparison to the effect BO and chronic lung rejection, these two factors do account for some discrepancy in survival among heart-lung recipients. Although debatable, Figure 3A shows that there is some concern that recipients less than 1 year of age have worse outcomes than older children who have undergone HLT (3,29). This is debatable because the number of HLTs that have occurred in children less than 1 year of age is very small. Outcomes for this age group could be highly dependent upon the experience of the transplant center as early outcomes are usually indicative of the success of the surgical procedure. Once beyond 2 years post-transplant infants with HLT have a similar risk of death as their older counterparts (3). The presence of Eisenmenger’s syndrome or congenital heart disease portends a worse outcome post HLT than for PPHN (Figure 3B). This may be due to the likelihood that patients with PPHN have less chronic disease and may have less deconditioning than those with Esienmenger’s and congenital heart disease.
Despite the significant barriers that may occur and the relatively dismal outcomes expected from HLT, this therapy still remains an important therapy for children with end-stage heart and lung disease. And, for most, HLT can offer improved QOL (30). Heart and lung function also significantly improve. The majority of DLT and HLT recipients experience significant improvements in lung function and exercise tolerance (31-34). The greatest improvement in lung function usually occurs in the first three months after DLT/HLT and slowly reaches a plateau at about one year, barring any concurrent significant complications that affect lung function (31). Normalization of pulmonary pressures, ventricular function and cardiac output are expected for patients who receive a HLT for pulmonary hypertension and cardiac disease.
Exercise tolerance improves greatly and allows most recipients to perform activities of daily living without limitation or need for supplemental oxygen or other supportive therapy. Over 80% of survivors at 1, 3, and 5 years post-transplant have no activity limitations (3). However, cardiopulmonary exercise testing reveals that maximum oxygen consumption is limited to 50-60% predicted at peak exercise (34). Deconditioning and a possible myopathy that is linked to the immunosuppressant regimen or other factors likely accounts for this limited exercise capacity, because cardiopulmonary reserve appears to be maintained. Recipients of heart, liver and kidney transplants have similar limitations on cardiopulmonary exercise, suggesting that factors other than graft function may account for the subnormal maximum oxygen consumption at peak exercise. In recipients of HLT versus DLT who were stable and otherwise well, there does not appear to be a difference in exercise capacity indicating that a healthy lung graft post-transplant does not appear to be the limiting organ. It is the cardiovascular response that appears to limit oxygen consumption at peak exercise (34).
QOL evaluations demonstrate that this procedure is perceived to be worthwhile to recipients. Most patients who have undergone transplant have been found to be happy with their decision to undergo the procedure (30). Interestingly, recent data have shown that 1-year QOL analysis for lung transplant recipients demonstrates a positive outcome for physical but not psychologic well-being. This demonstrates that transplant can confer physiologic improvements but the patient continues to have considerable medical burden in the post-transplant period with medical therapies, surveillance and fear of complications (35).
In children who have undergone thoracic transplantation, cognitive, academic and behavioral concerns arise after transplantation (36). This underscores the importance of psychosocial evaluation and counseling in the pre- and post-transplant period as this treatment can have effects on the patient and family. Despite these concerns about complications and outcomes, HLT can be an important therapy for those with end-stage heart and lung disease and success is determined by meticulous evaluation and surveillance.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Reitz BA. The first successful combined heart-lung transplantation. J Thorac Cardiovasc Surg 2011;141:867-9. [PubMed]
- Orens JB, Shearon TH, Freudenberger RS, et al. Thoracic organ transplantation in the United States, 1995-2004. Am J Transplant 2006;6:1188-97. [PubMed]
- Benden C, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Lung and Heart-Lung Transplantation Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:989-97. [PubMed]
- Kotsimbos T, Williams TJ, Anderson GP. Update on lung transplantation: programmes, patients and prospects. Eur Respir Rev 2012;21:271-305. [PubMed]
- Franke U, Wiebe K, Harringer W, et al. Ten years experience with lung and heart-lung transplantation in primary and secondary pulmonary hypertension. Eur J Cardiothorac Surg 2000;18:447-52. [PubMed]
- Goerler H, Simon A, Gohrbandt B, et al. Heart-lung and lung transplantation in grown-up congenital heart disease: long-term single centre experience. Eur J Cardiothorac Surg 2007;32:926-31. [PubMed]
- Goldstein BS, Sweet SC, Mao J, et al. Lung transplantation in children with idiopathic pulmonary arterial hypertension: an 18-year experience. J Heart Lung Transplant 2011;30:1148-52. [PubMed]
- Schwebel C, Pin I, Barnoud D, et al. Prevalence and consequences of nutritional depletion in lung transplant candidates. Eur Respir J 2000;16:1050-5. [PubMed]
- Data from the Organ Procurement and Transplantation Network (OPTN). As of December 13, 2013. Available online: http://optn.transplant.hrsa.gov
- Canter CE, Shaddy RE, Bernstein D, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;115:658-76. [PubMed]
- Dobbels F, Vanhaecke J, Desmyttere A, et al. Prevalence and correlates of self-reported pretransplant nonadherence with medication in heart, liver, and lung transplant candidates. Transplantation 2005;79:1588-95. [PubMed]
- International Society for Heart and Lung Transplantation (ISHLT) Registry Quarterly Reports for Heart/Lung in North America. As of December 4, 2013.
- Children’s Hospital of Pittsburgh of UPMC, Heart and Lung Transplant. Internal data as of December 15, 2013.
- Wuyts WA, Herijgers P, Budts W, et al. Extensive dissection of the pulmonary artery treated with combined heart-lung transplantation. J Thorac Cardiovasc Surg 2006;132:205-6. [PubMed]
- Talbot SM, Taub RN, Keohan ML, et al. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg 2002;124:1145-8. [PubMed]
- van der Bom T, Zomer AC, Zwinderman AH, et al. The changing epidemiology of congenital heart disease. Nat Rev Cardiol 2011;8:50-60. [PubMed]
- Feinstein JA, Benson DW, Dubin AM, et al. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol 2012;59:S1-42. [PubMed]
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297-310. [PubMed]
- Trulock EP, Ettinger NA, Brunt EM, et al. The role of transbronchial lung biopsy in the treatment of lung transplant recipients. An analysis of 200 consecutive procedures. Chest 1992;102:1049-54. [PubMed]
- Aboyoun CL, Tamm M, Chhajed PN, et al. Diagnostic value of follow-up transbronchial lung biopsy after lung rejection. Am J Respir Crit Care Med 2001;164:460-3. [PubMed]
- Chakinala MM, Ritter J, Gage BF, et al. Yield of surveillance bronchoscopy for acute rejection and lymphocytic bronchitis/bronchiolitis after lung transplantation. J Heart Lung Transplant 2004;23:1396-404. [PubMed]
- Bankier AA, Van Muylem A, Knoop C, et al. Bronchiolitis obliterans syndrome in heart-lung transplant recipients: diagnosis with expiratory CT. Radiology 2001;218:533-9. [PubMed]
- Lee ES, Gotway MB, Reddy GP, et al. Early bronchiolitis obliterans following lung transplantation: accuracy of expiratory thin-section CT for diagnosis. Radiology 2000;216:472-7. [PubMed]
- Hayes D Jr, Galantowicz M, Hoffman TM. Combined heart-lung transplantation: a perspective on the past and the future. Pediatr Cardiol 2013;34:207-12. [PubMed]
- Spira A, Gutierrez C, Chaparro C, et al. Osteoporosis and lung transplantation: a prospective study. Chest 2000;117:476-81. [PubMed]
- Leblond V, Sutton L, Dorent R, et al. Lymphoproliferative disorders after organ transplantation: a report of 24 cases observed in a single center. J Clin Oncol 1995;13:961-8. [PubMed]
- Kurland G, Orenstein DM. Lung transplantation and cystic fibrosis: the psychosocial toll. Pediatrics 2001;107:1419-20. [PubMed]
- Scott JP, Higenbottam TW, Sharples L, et al. Risk factors for obliterative bronchiolitis in heart-lung transplant recipients. Transplantation 1991;51:813-7. [PubMed]
- Deuse T, Sista R, Weill D, et al. Review of heart-lung transplantation at Stanford. Ann Thorac Surg 2010;90:329-37. [PubMed]
- Choong CK, Meyers BF. Quality of life after lung transplantation. Thorac Surg Clin 2004;14:385-407. [PubMed]
- Egan TM, Detterbeck FC, Mill MR, et al. Improved results of lung transplantation for patients with cystic fibrosis. J Thorac Cardiovasc Surg 1995;109:224-34; discussion 234-5. [PubMed]
- Levy RD, Ernst P, Levine SM, et al. Exercise performance after lung transplantation. J Heart Lung Transplant 1993;12:27-33. [PubMed]
- Williams TJ, Patterson GA, McClean PA, et al. Maximal exercise testing in single and double lung transplant recipients. Am Rev Respir Dis 1992;145:101-5. [PubMed]
- Levy RD, Ernst P, Levine SM, et al. Exercise performance after lung transplantation. J Heart Lung Transplant 1993;12:27-33. [PubMed]
- Finlen Copeland CA, Vock DM, Pieper K, et al. Impact of lung transplantation on recipient quality of life: a serial, prospective, multicenter analysis through the first posttransplant year. Chest 2013;143:744-50. [PubMed]
- Brosig C, Hintermeyer M, Zlotocha J, et al. An exploratory study of the cognitive, academic, and behavioral functioning of pediatric cardiothoracic transplant recipients. Prog Transplant 2006;16:38-45. [PubMed]


