Expression of estrogen receptor beta (ERβ) and its prognostic value in pleural mesothelioma
Introduction
Malignant mesothelioma is an aggressive tumor which may originate in the mesothelial tissue of either pleural or abdominal cavities, the tunica vaginalis or pericardium. Diagnosis occurs more frequently during advanced stages, appearing as an acute admission to hospital due to breathlessness, chest pain and unilateral pleural effusion (1) with a negative prognosis for the majority of patients despite treatment (2-6). Statistics related to its morbidity and mortality rates are limited; in 2013, 50,400 new cases of malignant pleural mesothelioma (MPM) were estimated, and 33,700 (66.9%) deaths due to this disease (7,8).
At the United States, there is a medium rate of incidence with close to 3,300 new cases reported and 2,700 deaths every year (9). In Mexico, this tumor is ranked 34th among malignant neoplasms (10), during the period of 1979 to 2000 a study reported 793 deaths by MPM in the country, of which 62% were male patients and 38% female. These statistics concur with those collected at the Instituto Nacional de Enfermedades Respiratorias “Ismael Cosío Villegas” (INER), a third-level care center specialized in pulmonary pathology, including lung and pleural cancer, which are representative of the epidemiology within the country’s central region. Between 1991 and 2007 a total of 247 new diagnoses were reported (74.7% male) (11), followed by a significant increase in incidence between 2006 and 2009 of 149 new cases (71.1% male) (12). In both studies, the age at presentation varied between 51 and 70 years old and exposure to asbestos of any kind was documented in up to 91% of cases (11,12).
To date, there is no malignancy that has a more causal relation with a defined carcinogen than MPM with asbestos (up to 90%), after an approximate 30-year latency period of exposure (13-16). According to the International Agency for Research on Cancer there are at least 13 cohort studies and 18 case-control studies probing the relation between asbestos exposure and mesothelioma (17), however, the latency time is long but the survival at diagnosis is short, regardless of the exposure time (18-20). Unlike the reduction in the use of asbestos in developed countries, Asia and Latin America has become an increasingly common trend resulting in a continuous growth in the incidence of mesothelioma in the next 10 to 20 years (21-24). Environmental exposure could be also due to other asbestiform fibers (such as Libby amphibole and Fluoro-edenite) into air by routine human activities or natural weathering processes, this exposure may vary across the area by task and according to the spatial distribution of contamination, soil, vegetation type, and environmental conditions (25,26). Besides the asbestos exposure, some patients do not report known exposure (27,28), so other types of factors have been studied such as different mineral fibers other than asbestos, radiation, chronic serosal inflammatory conditions, simian virus 40, BAP-1 cancer predisposition syndrome, contributing to the development of MPM (29-31). In different studies, the factors involved in carcinogenesis already mentioned above result in alteration of immunocompetent cells to result in a decline of tumoral immunity. Asbestos can induce chronic inflammation due to the production of reactive oxygen/nitrogen (32) more pathogenic in in vivo than in in vitro by macrophage activation (33) that results in increased NF-κB activity, a signaling pathway that plays a role orchestrating the inflammatory response as well as cell proliferation (34). This state of inflammation has tried to be demonstrated in different ways such as the verification of the presence of antinuclear autoantibodies (35) or elevation of biomarkers such as serum mesothelin in those exposed to asbestos fibers (36).
MPM is hard to stage due to the lack of consensus on the staging system, however, particularly among patients that do not undergo surgery, it is a common practice to use the American Joint Committee on Cancer (AJCC) TNM (tumor, nodes, metastasis staging system) (37-39).
A biopsy to have a definitive diagnosis is of high importance to begin treatment expeditiously (40-42). Recently, the role of estrogen receptor expression in different human tumors has remained controversial (43), however, in malignant mesothelial tissue there is evidence of its usefulness as a prognostic factor (44-46). Considering that this tumor is less frequent in women and that they have a more favorable prognosis (47), it may be hypothesized that development of this tumor could be related to the expression of estrogen receptors (48). A study that analyzed 78 samples of MPM tissue and 21 samples of normal pleural tissue and demonstrated that there was no expression of ERα in any of the samples, either malignant or normal. On the other hand, both tissue types presented expression of ERβ. This same study demonstrated that overexpression of this receptor is independently related with a better survival prognosis, most notably in the epithelioid histological subtype. Moreover, it was demonstrated that expression of this receptor inhibits the growth of tumor cells promoting the expression of proteins p21 and p27 and inhibiting the expression of cyclin B1 (49). It has even been possible to link the expression of ERβ to the cell’s metabolic state, where a higher lactate concentration in the intracellular space results in a greater expression of ERβ (50).
Conversely, two studies have demonstrated that using a selective agonist of ERβ, in this case, a molecule known as KB9520 decreases the growth of MPM cells in both in vitro and in murine models (50,51). In addition, there is evidence that the selective agonism of this receptor could increase the sensitivity to the antitumor treatment. In one study an increased sensitivity to cisplatin was reported in vitro together with a protective effect to this cytotoxic agent in normal mesothelial cells (51-53). In a similar fashion, another study produced sensitivity to the use of the EGFR tyrosine-kinase inhibitor gefitinib, which seems to diminish its rate of internalization when the agonist KB9520 was added to an in vitro model (54). The aim of this study was to assess the response rate to first-line chemotherapy in malignant pleural mesothelioma with an expression of ERβ.
Methods
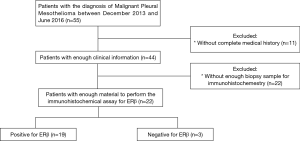
A retrospective study was performed at the Thoracic Oncology and Pathological Anatomy departments at INER, in Mexico City. The study design was approved by INER’s Institutional Ethics Board in accordance with the Declaration of Helsinki, Fortaleza Brazil 2013 (approval document: C56-18). Patients older than 18 years old with a histopathological diagnosis of malignant pleural mesothelioma were included, diagnosed between December 2013 and June 2016, with at least one chest imaging study prior to the start of chemotherapy. Exclusion criteria were underage patients, those that underwent resection or radiotherapy with curative intent prior to chemotherapy, and those without a complete medical history or enough paraffin blocks to perform immunohistochemical studies as shown in Figure 1. The primary endpoint was the rate of response to first-line chemotherapy within the first 6 months, and the secondary endpoints were the percentage of resectability, progression-free survival (PFS), and overall survival (OS).
The following clinical and pathological variables were determined: gender, age, expositional, mesothelioma pattern, TNM clinical stage, first-line chemotherapy scheme, response as per RECIST 1.1, OS and PFS.
In addition, immunohistochemical analysis was performed on the biopsy samples to determine the expression of ERβ by our pathologist, who was blinded to the treatment received by the patient, as well as his survival rate. An immunohistochemical assay with a murine monoclonal antibody aimed at the region C-terminal of isoform 1 of the human ERβ was used (GTX47720, PPG5/10, GeneTex Inc., Indio, CA, USA). An automated, standardized, immunohistochemistry VENTANA Benchmark XT system (Ventana Medical Systems, Roche Inc., Tucson, AZ, USA) was also used. To determine the degree of positive staining by direct observation, a qualitative scale was used as follows: 1+ for weak staining, 2+ for intense staining, and 3+ for very intense staining; a percentage of cells that were stained with antibody was also calculated. High and moderate expression of ERβ, considered at the same group, was determined when more than 50% of cells were stained with an intensity greater than 1+. SPSS software version 24.0 (IBM software, Armonk, NY, USA) was used for analysis. The variables were expressed as the median values, as well as total values and percentages. OS and PFS were graphed using a Kaplan-Meier plot. The criterion for statistical significance was P<0.05. The immunohistochemical and all the financial related issues were absorbed by the investigation group from the study.
Results
A total of 55 patients were identified with a diagnosis of MPM starting December 2013. Of these, 37 patients were male (67.3%) and 18 were female (32.7%). The average age in years was 64.1 (standard deviation 9.94) with a range of 41 to 84 years. Only 44 patients (80%) had enough information in either paper or electronic charts at INER in order to be considered for the statistical analysis.
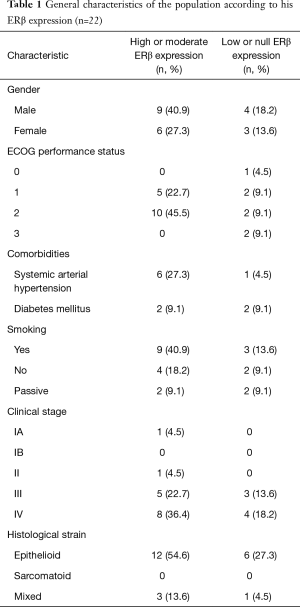
Regarding the histopathological characteristics of the tumors, 42 out of the 44 cases had an epithelioid histology (95.5%) with the rest being of mixed histology (4.5%). There were no cases of only sarcomatoid histology. Out of the 44 cases, only 22 (50%) had enough material to perform the immunohistochemical assay to determine the expression of estrogen receptor beta (ERβ). According to the TNM classification the 22 cases had the following clinical stage distribution at the moment of the diagnosis: stage IV 54.6%, stage III 36.3%, stages II and IA each with 4.5%. Amongst the patients that were statistically analyzed as per their ERβ expression, exposure to asbestos was only documented in 9 out of 22 cases (40.9%). It was possible to perform surgical resection after treatment with chemotherapy in 4 patients (18%) and 3 patients received radiotherapy (14%). The median PFS in this population was 9.85 months, while the median OS was 14.35 months. Table 1 lists the characteristics of the population that was included in the ERβ expression analysis.
Full table
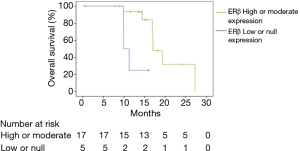
Of the 22 tissue samples available for ERβ immunohistochemical analysis, 19 were positive for this receptor (86.4%) and in 15 of these 100% of cells were stained with different degrees of intensity (68.2%). Table 2 shows the response to first-line chemotherapy as well as the possibility to provide definitive treatment to patients, with either chemotherapy or radiotherapy, after chemotherapy treatment. Table 2 also lists the PFS and OS as they relate to ERβ expression. The response to treatment as by RECIST 1.1, 12 (54.6%) had a partial response, 5 (22.7%) had stable disease, and 3 (13.6%) had progression. None of the patients had a complete response. Of the patients who had a partial response, 9 (75%) patients had a high or moderate degree of ERβ expression in tumor cells, and 3 (25%) had a low or null degree of expression. According to the criterion used to define high and moderate ERβ expression group (positive staining in 50% of cells), it was observed that patients with partial response after first-line chemotherapy and high or moderate ERβ expression were 41% of the population (9 patients) while those with low or null ERβ expression were 14% (3 patients). No patient had a full response. The percentage of resectability after chemotherapy in patients with either high or moderate and low or null ERβ expression was 9% in both groups. Neither the difference in partial response nor the percentage of resectability achieved statistical significance. The median PFS for patients with high or moderate ERβ expression was 12.2 months, compared to 9.3 months amongst those with low or null expression (P=0.67, 95% CI, 4.8–12.9). The median OS had a statistical tendency to be greater in patients with high or moderate ERβ expression by 9.2 months since the difference of the medians was 19.5 and 10.3 months for patients with high or moderate and low or null expression, respectively (P=0.054, 95% CI, 9.79–10.01). This relationship is shown in Figure 2.

Full table
Exploratory analyses to identify risk factors for OS were performed with the use of a multivariate logistic-regression model (age, gender, ECOG performance status, smoking index, histological strain, clinical stage, degree of ERβ expression and chemotherapy regimen), with no significant predictors for OS were found in this analysis.
Discussion
The general characteristics of the population agree with previous reports described in the literature. The average age in years was 64.1 (range, 41 to 84), being the stage IV the most prevalent clinical stage at the moment of the diagnosis (54.5%). Since most diagnoses are made in advanced stages, it becomes of broad interest the identification of non-invasive molecular markers for an early diagnosis. The most studied biomarker is mesothelin, characterized by a good specificity, but it has low sensitivity. Other protein markers had reported interesting results such as the HMGB1 (55) and microRNAs expression (56) as promising diagnostic biomarkers, notwithstanding the above, none of the markers available today are sufficiently reliable to be used in the surveillance of subjects exposed to asbestos or in the early detection of MPM. Exposure to asbestos was only documented in 41% of the studied population, which represents a lower percentage of what is described in the literature. This may be due to underestimation while questioning the patient or to unknown exposure to these compounds (mainly in construction materials), that is subsequently not reported. Finally, tobacco use was reported by slightly more than half of the analyzed patients. Although there is a greater expression of ERβ in men, this may be due to the prevalence of MPM in men without a clear relationship between the degree of ERβ expression and gender. It is noteworthy to see that those patients with worse ECOG had higher expression of ERβ. Regarding the histological strain, those with mixed subtype showed lower expression of ERβ possibly explained due to the aggressiveness and poor differentiation of this subtype.
There is an apparent relationship between the rate of response to treatment and a high or moderate ERβ expression, although it did not achieve statistical significance. It is important to mention that no direct comparison was made between the type of chemotherapy used as first-line of treatment and the obtained response. No relationship was found between resectability after chemotherapy treatment and ERβ expression, or a statistically significant relationship between ERβ expression and PFS. However, a tendency was found that links a better OS to patients with high or moderate ERβ expression. This is correlated with studies reported in the literature where the expression of this receptor leads to a better prognosis in OS.
It is important to mention that group of investigators decided to use the PPG5/10 antibody due to validations previously made by Saunders et al., 2002 (57), Shaaban et al., 2008 (58) and Wu et al., 2012 (59), however, a year later from the processing and collection of our samples, Andersson et al., 2017 (60) and Nelson et al., 2017 (61) published studies where the validity of different anti-ERβ antibodies was compared generating controversy about the specificity of the PPG5/10 antibody. This is of relevance since due to current financial issues of the research group, our interest of verifying ERβ expression through the use of other antibodies such as PPZ0506 or CWK-F12 is limited, being aware of the need for a future prospective study using the antibodies of interest already mentioned.
This study highlights that the response to treatment is apparently not related to ERβ expression. However, studies that showed an increased response to treatment with either cisplatin or a tyrosine-kinase inhibitor used an agonist specific to this receptor. Therefore, with our results, it may not be possible to discard the fact that, apart from being a potential prognostic marker, ERβ expression could have predictive value for treatment with a selective agonist (51,54). The main limitations of this work include the sample size which is modest compared to other literature reports that analyzed up to 78 patients (50,51,54). This imposes a limitation to the statistical power of our findings despite being partially in agreement with reports in the literature and in other studies.
Some other limitations were the inclusion of a single hospital, the loss of patients due to incomplete medical charts, and the lack of sufficient tissue samples, which resulted in 60% of patients diagnosed with mesothelioma during the study period not being included in the final analysis of ERβ expression. Finally, the retrospective nature of the study impacts the ability to generalize the results.
Amongst the strengths of this study, stands out the fact that this is the first time that ERβ expression in advanced mesothelioma is studied in Mexico. In addition, this study linked ERβ expression to patient prognosis and showed results that agree with international literature reports. The inclusion of more patients to this cohort and expansion into a prospective study will improve its statistical power, which would certainly demonstrate the survival benefit with a degree of significance greater than just a tendency. Moreover, it would be possible to include in a future study the use of a selective agonist of ERβ concomitant to chemotherapy in order to compare the response to treatment in vivo in a clinical setting, and not just in vitro as reported so far in the literature (51,54).
Conclusions
A high or moderate expression of ERβ as measured by immunohistochemistry was related to a higher response to chemotherapy treatment in patients with advanced malignant pleural mesothelioma, although this result was not statistically significant. It was not possible to link the expression of ERβ with a higher rate of resectability in patients with advanced malignant pleural mesothelioma.
A tendency was found for higher OS in patients with advanced malignant pleural mesothelioma and high or moderate ERβ expression.
Acknowledgements
The authors would like to thank the redaction team from Boehringer Ingelheim Mexico for valuable and critical review of our manuscript. The authors also thank the Department of Pathology and the Department of Thoracic Oncology from the “Instituto Nacional de Enfermedades Respiratorias, Ismael Cosío” for providing the clinical files of the patients studied.
Footnote
Conflicts of Interest: Dr. Jeronimo Rafael Rodríguez-Cid has educational, investigational and advice relations with MSD, Bristol Myers, Roche, Takeda, Amgen, Abvie, Aztra Zeneca, Boehringer Ingelheim, Pfizer, Celgen, Novartis and Bayer; Dr. Orlando García-Acevedo works today for Aztra Zeneca as MSL; Dr. Jorge Arturo Alatorre-Alexander has educational, investigational and advice relations with MSD, Bristol Myers, Roche, Takeda, Aztra Zeneca, Boehringer Ingelheim and Pfizer. The other authors have no conflicts of interest to declare.
Ethical Statement: The study design was approved by INER’s Institutional Ethics Board in accordance with the Declaration of Helsinki, Fortaleza Brazil 2013 (approval document: C56-18).
References
- Tolani B, Acevedo L, Hoang N, et al. Heterogeneous Contributing Factors in MPM Disease Development and Progression: Biological Advances and Clinical Implications. Int J Mol Sci 2018;19:238. [Crossref] [PubMed]
- Oehl K, Vrugt B, Opitz I, et al. Heterogeneity in Malignant Pleural Mesothelioma. Int J Mol Sci 2018;19:1603. [Crossref] [PubMed]
- Damhuis RA, van Meerbeeck J. International trends in the clinical epidemiology of malignant pleural mesothelioma. J Thorac Dis 2018;10:1147-8. [Crossref] [PubMed]
- Rahouma M, Aziz H, Ghaly G, et al. Survival in Good Performance Malignant Pleural Mesothelioma Patients; Prognostic Factors and Predictors of Response. Asian Pac J Cancer Prev 2017;18:2073-8. [PubMed]
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [Crossref] [PubMed]
- Chailleux E, Dabouis G, Pioche D, et al. Prognostic Factors in Diffuse Malignant Pleural Mesothelioma. Chest 1988;93:159-62. [Crossref] [PubMed]
- Vos T, Barber R, Bell B, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743-800. [Crossref] [PubMed]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. [Crossref] [PubMed]
- Teta M, Mink P, Lau E, et al. US mesothelioma patterns 1973–2002: indicators of change and insights into background rates. Eur J Cancer Prev 2008;17:525-34. [Crossref] [PubMed]
- Fernández S, Sánchez M, González M, et al. Perfil Epidemiológico De Los Tumores Malignos En México. México: Secretaría de salud, 2011.
- Aguilar-Madrid G, Juárez-Pérez C, Markowitz S, et al. Globalization and the Transfer of Hazardous Industry: Asbestos in Mexico, 1979–2000. Int J Occup Environ Health 2003;9:272-9. [Crossref] [PubMed]
- Echegoyen-Carmona R. Epidemiología clínica del mesotelioma pleural maligno en el INER. Neumol Cir Torax 2012;71:219-25.
- Robinson BW, Musk A, Lake R. Malignant mesothelioma. Lancet 2005;366:397-408. [Crossref] [PubMed]
- Selikoff IJ, Hammond E, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980;46:2736-40. [Crossref] [PubMed]
- Cox LA Jr. Biological mechanisms of non-linear dose-response for respirable mineral fibers. Toxicol Appl Pharmacol 2018;361:137-44. [Crossref] [PubMed]
- Woolhouse I, Bishop L, Darlison L, et al. BTS guideline for the investigation and management of malignant pleural mesothelioma. BMJ Open Respir Res 2018;5:e000266. [Crossref] [PubMed]
- International Agency for Research on Cancer. IARC Monographs Volume 100C Asbestos (Chrysotile, Amosite, Crocidolite, Tremolite, Actinolite and Anthophyllite) – IARC [Internet]. Monographs.iarc.fr. 2018 [cited 20 January 2019]. Available online: https://monographs.iarc.fr/iarc-monographs-volume-100c-asbestos-chrysotile-amosite-crocidolite-tremolite-actinolite-and-anthophyllite/
- Bianchi C, Giarelli L, Grandi G, et al. Latency periods in asbestos-related mesothelioma of the pleura. Eur J Cancer Prev 1997;6:162-6. [PubMed]
- Mowé G, Gylseth B, Hartveit F, et al. Occupational asbestos exposure, lung-fiber concentration and latency time in malignant mesothelioma. Scand J Work Environ Health 1984;10:293-8. [Crossref] [PubMed]
- Chouaid C, Assié J, Andujar P, et al. Determinants of malignant pleural mesothelioma survival and burden of disease in France: a national cohort analysis. Cancer Medicine 2018;7:1102-9. [Crossref] [PubMed]
- Tsou JA, Galler J, Wali A, et al. DNA methylation profile of 28 potential marker loci in malignant mesothelioma. Lung Cancer 2007;58:220-30. [Crossref] [PubMed]
- Waller DA. The management of malignant pleural mesothelioma in the USA 2004-13—a decade of lost opportunity? J Thorac Dis 2018;10:S1044-6. [Crossref] [PubMed]
- Katzman D, Sterman D. Updates in the diagnosis and treatment of malignant pleural mesothelioma. Curr Opin Pulm Med 2018;24:319-26. [Crossref] [PubMed]
- Blyth KG, Murphy D. Progress and challenges in Mesothelioma: From bench to bedside. Respir Med 2018;134:31-41. [Crossref] [PubMed]
- Harper M, Butler C, Berry D, et al. Where occupation and environment overlap: US forest service worker exposure to libby amphibole fibers. J Occup Environ Hyg 2015;12:D47-53. [Crossref] [PubMed]
- Ledda C, Pomara C, Bracci M, et al. Natural carcinogenic fiber and pleural plaques assessment in a general population: A cross-sectional study. Environ Res 2016;150:23-9. [Crossref] [PubMed]
- Marinaccio A, Corfiati M, Binazzi A, et al. The epidemiology of malignant mesothelioma in women: gender differences and modalities of asbestos exposure. Occup Environ Med 2018;75:254-62. [Crossref] [PubMed]
- Moreno de la Santa P, Butchart E. Mesotelioma Pleural Maligno. Pneuma 2006;4:41-50.
- Attanoos RL, Churg A, Galateau-Salle F, et al. Malignant Mesothelioma and Its Non-Asbestos Causes. Arch Pathol Lab Med 2018;142:753-60. [Crossref] [PubMed]
- Pistolesi M, Rusthoven J. Malignant pleural mesothelioma: update, current management, and newer therapeutic strategies. Chest 2004;126:1318-29. [Crossref] [PubMed]
- Kinoshita Y, Takasu K, Yuri T, et al. Two cases of malignant peritoneal mesothelioma without asbestos exposure: cytologic and immunohistochemical features. Ann Diagn Pathol 2013;17:99-103. [Crossref] [PubMed]
- Matsuzaki H, Maeda M, Lee S, et al. Asbestos-Induced Cellular and Molecular Alteration of Immunocompetent Cells and Their Relationship with Chronic Inflammation and Carcinogenesis. J Biomed Biotechnol 2012;2012:492608. [Crossref] [PubMed]
- Donaldson K, Brown G, Brown D, et al. Inflammation generating potential of long and short fibre amosite asbestos samples. Br J Ind Med 1989;46:271-6. [PubMed]
- Haegens A, Barrett T, Gell J, et al. Airway Epithelial NF- B Activation Modulates Asbestos-Induced Inflammation and Mucin Production In Vivo. J Immunol 2007;178:1800-8. [Crossref] [PubMed]
- Ledda C, Caltabiano R, Loreto C, et al. Prevalence of anti-nuclear autoantibodies in subjects exposed to natural asbestiform fibers: a cross-sectional study. J Immunotoxicol 2018;15:24-8. [Crossref] [PubMed]
- Kodavanti UP, Andrews D, Schladweiler M, et al. Early and Delayed Effects of Naturally Occurring Asbestos on Serum Biomarkers of Inflammation and Metabolism. J Toxicol Environ Health A 2014;77:1024-39. [Crossref] [PubMed]
- Enewold L, Sharon E, Thomas A. Patterns of care and survival among patients with malignant mesothelioma in the United States. Lung Cancer 2017;112:102-8. [Crossref] [PubMed]
- Hoda MA, Ploenes T, Aigner C. Malignant pleural mesothelioma—the impact of globalization on rare diseases. J Thorac Dis 2018;10:638-40. [Crossref] [PubMed]
- Abdel-Rahman O. Challenging a dogma; AJCC 8th staging system is not sufficient to predict outcomes of patients with malignant pleural mesothelioma. Lung Cancer 2017;113:128-33. [Crossref] [PubMed]
- Szolkowska M, Blasinska-Przerwa K, Knetki-Wroblewska M, et al. Malignant pleural mesothelioma: main topics of American Society of Clinical Oncology clinical practice guidelines for diagnosis and treatment. J Thorac Dis 2018;10:S1966-70. [Crossref] [PubMed]
- McCambridge AJ, Napolitano A, Mansfield A, et al. Progress in the Management of Malignant Pleural Mesothelioma in 2017. J Thorac Oncol 2018;13:606-23. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr K, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v31-9. [Crossref] [PubMed]
- Boutin C, Rey F, Gouvernet J, et al. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993;72:394-404. [Crossref] [PubMed]
- Ordóñez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 2003;27:1031-51. [Crossref] [PubMed]
- Manente AG, Pinton G, Zonca S, et al. KDM6B histone demethylase is an epigenetic regulator of estrogen receptor β expression in human pleural mesothelioma. Epigenomics 2016;8:1227-38. [Crossref] [PubMed]
- Jennings CJ, O'Grady A, Cummins R, et al. Sustained Expression of Steroid Receptor Coactivator SRC-2/TIF-2 is Associated with Better Prognosis in Malignant Pleural Mesothelioma. J Thorac Oncol 2012;7:243-8. [Crossref] [PubMed]
- Chua TC, Yao P, Akther J, et al. Differential Expression of Ki-67 and Sex Steroid Hormone Receptors Between Genders in Peritoneal Mesothelioma. Pathol Oncol Res 2009;15:671-8. [Crossref] [PubMed]
- Pinton G, Moro L. Expression and therapeutic significance of estrogen receptor β in malignant pleural mesothelioma. Future Sci OA 2017;3:FSO175. [Crossref] [PubMed]
- Pinton G, Brunelli E, Murer B, et al. Estrogen Receptor-Beta Affects the Prognosis of Human Malignant Mesothelioma. Cancer Res 2009;69:4598-604. [Crossref] [PubMed]
- Manente AG, Pinton G, Zonca S, et al. Intracellular lactate-mediated induction of estrogen receptor beta (ERβ) in biphasic malignant pleural mesothelioma cells. Oncotarget 2015;6:25121-34. [Crossref] [PubMed]
- Pinton G, Manente A, Daga A, et al. Agonist activation of estrogen receptor beta (ERβ) sensitizes malignant pleural mesothelioma cells to cisplatin cytotoxicity. Mol Cancer 2014;13:227. [Crossref] [PubMed]
- Pinton G, Zonca S, Manente A, et al. SIRT1 at the crossroads of AKT1 and ERβ; in malignant pleural mesothelioma cells. Oncotarget 2016;7:14366-79. [Crossref] [PubMed]
- Manente AG, Valenti D, Pinton G, et al. Estrogen receptor β activation impairs mitochondrial oxidative metabolism and affects malignant mesothelioma cell growth in vitro and in vivo. Oncogenesis 2013;2:e72. [Crossref] [PubMed]
- Pinton G, Thomas W, Bellini P, et al. Estrogen Receptor β Exerts Tumor Repressive Functions in Human Malignant Pleural Mesothelioma via EGFR Inactivation and Affects Response to Gefitinib. PLoS One 2010;5:e14110. [Crossref] [PubMed]
- Ledda C, Senia P, Rapisarda V. Biomarkers for Early Diagnosis and Prognosis of Malignant Pleural Mesothelioma: The Quest Goes on. Cancers (Basel) 2018;10. [Crossref] [PubMed]
- Martínez-Rivera V, Negrete-García MC, Ávila-Moreno F, et al. Secreted and Tissue miRNAs as Diagnosis Biomarkers of Malignant Pleural Mesothelioma. Int J Mol Sci 2018;19. [Crossref] [PubMed]
- Saunders PT, Millar M, Williams K, et al. Expression of oestrogen receptor beta (ERβ1) protein in human breast cancer biopsies. Br J Cancer 2002;86:250-6. [Crossref] [PubMed]
- Shaaban AM, Green A, Karthik S, et al. Nuclear and Cytoplasmic Expression of ER 1, ER 2, and ER 5 Identifies Distinct Prognostic Outcome for Breast Cancer Patients. Clinical Cancer Research 2008;14:5228-35. [Crossref] [PubMed]
- Wu X, Subramaniam M, Negron V, et al. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem 2012;113:711-23. [Crossref] [PubMed]
- Andersson S, Sundberg M, Pristovsek N, et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun 2017;8:15840. [Crossref] [PubMed]
- Nelson AW, Groen A, Miller J, et al. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol Cell Endocrinol 2017;440:138-50. [Crossref] [PubMed]