Anaplastic lymphoma kinase inhibitors in non-small cell lung cancer patients with brain metastases: a meta-analysis
Introduction
Central nervous system (CNS) metastases are a common complication in a wide range of cancers, and they are particularly common among patients with non-small cell lung cancer (NSCLC), developing in approximately 30% of patients with advanced stage adenocarcinomas (1). Patients with brain metastases (BMs) experience significant morbidity and poor prognosis. Local therapies have been the primary approaches to the treatment of patients with BMs including surgery, whole brain radiation therapy (WBRT), and stereotactic radiosurgery (SRS). The efficacy of systemic treatment is limited due to the blood-brain barrier which prevents drugs reaching the brain parenchyma. Even the physical structure of blood-brain barrier is disrupted by BMs or radiotherapy, systemic therapies are often expelled by efflux transporters (2,3).
Anaplastic lymphoma kinase (ALK) rearrangement is responsible for approximately 5% of NSCLC, with EML4-ALK translocation as the most common one (4). As a therapeutic target of NSCLC, ALK rearrangement is usually associated with younger age, never or light smoking history and adenocarcinoma histology (5,6). Incidence of BMs is higher in patients whose cancers harbor ALK rearrangement, in whom up to 50 to 60 percent will develop BMs over the course of their disease (7-9), therefore, agents with good blood-brain barrier penetration are needed for this patients setting. Crizotinib is the first approved anti-ALK tyrosine kinase inhibitor which significantly improved the survival and tumor response in ALK-positive NSCLC patients when compared with standard chemotherapy (10-12). However, crizotinib is associated with poor intracranial disease control with up to 60% of patients developing BMs during treatment (13). Recently, next-generation ALK inhibitors has demonstrated significant CNS activity in the upfront and post-crizotinib settings including ceritinib, alectinib, brigatinib, and lorlatinib, indicating anti-ALK treatment is an acceptable option for patients with ALK-positive NSCLC and BMs (14-28). So far, the activity of ALK inhibitors has not been systematically investigated in patients with NSCLC-BMs. Therefore, we reviewed all the publications and conducted a meta-analysis to assess the efficacy of ALK inhibitors in NSCLC-BMs.
Methods
Search strategy
A comprehensive search for studies published in English was performed in PubMed, Cochrane library, Web of Science, and Embase from the inception dates to July 17, 2018, using the keywords “non-small cell lung cancer” OR “NSCLC” AND “brain metastases” OR “central nervous system metastases” AND “anaplastic lymphoma kinase” OR “ALK” AND “crizotinib” OR “ceritinib” OR “LDK378” OR “alectinib” OR “CH5424802” OR “brigatinib” OR “AP26113” OR “lorlatinib” OR “PF-06463922”. This analysis was performed in accordance with the PRISMA statement (29).
Inclusion and exclusion criteria
Trials were selected based on the following inclusion criteria: articles or supplements that evaluate anti-ALK agents in treatment of NSCLC patients with BMs, articles with or without report of ALK status will be included; studies including 1 or all of the following information: intracranial objective response rate (iORR), intracranial disease control rate (iDCR), intracranial duration of response (iDOR), intracranial progression-free survival (iPFS), and OS. All investigational studies were acceptable including single-arm studies, retrospective studies, and randomized studies. Letters, editorials, expert opinions, case reports, duplicate publications, and reviews should be excluded as well as the studies without usable data. Discrepancies between two reviewers were solved by discussion and consensus.
Quality assessment
The methodological quality for the included studies was assessed independently by 2 researchers (Z Zhang and H Guo) based on Cochrane risk-of-bias criteria for randomized studies and Newcastle-Ottawa Scale (NOS) for non-randomized studies. The researchers resolved disagreement by discussion. We evaluated methodological quality as low, high, or unclear risk of bias, which included the randomization sequence generation, allocation concealment, blinding of participants and personnel as well as outcome assessment, incomplete outcome data, selective reporting, and other bias.
Data extraction
Two researchers (Y Lu and W Hao) independently extracted the following information from each study: lead author; publication year; country of origin; study design, treatment strategy, number of patients, dose, ALK status, previous treatment, and efficacy parameters (iORR, iDCR, or iPFS). Disagreements were also solved by discussion and consensus.
Statistical analysis
All statistical analyses were performed by R version 3.5.0. Statistical heterogeneity among studies was checked using the Q test and I2 statistic. A high value of I2 indicated a higher probability of the existence of heterogeneity (I2=0–25%, no heterogeneity; I2=25–50%, moderate heterogeneity; I2=50–75%, large heterogeneity; and I2=75–100%, extreme heterogeneity). A fixed effect model was used when P value greater than 0.10, otherwise, a random effects model was adopted. Potential publication bias was investigated through funnel plots and Egger’s test. A two-sided P value <0.05 was considered statistically significant.
Results
Studies retrieved and characteristics
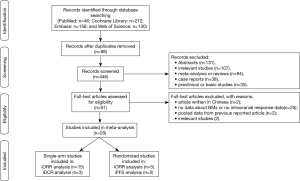
The study selection process was shown in Figure 1. A total of 544 results were identified from the searches in PubMed (n=46), Cochrane Library (n=212), Embase (n=156), and Web of Science (n=130). After removing 98 duplicated records, we then excluded 395 records including abstracts, irrelevant studies, meta-analysis or reviews, case reports, protocols or study designs, and preclinical or basic studies. The remaining 51 full-text articles were reviewed in detail, and 31 of them were also removed because the studies included articles which were not relevant, not written in English, pooled data analysis from previous reported articles, or the data for intracranial efficacy was not available.
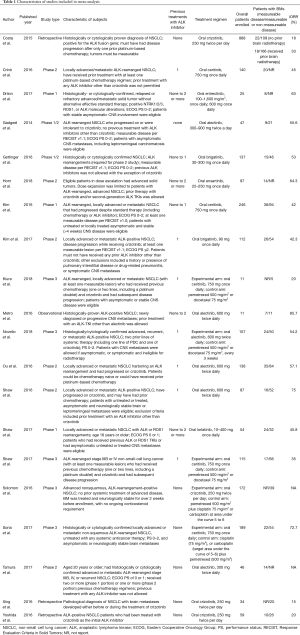
Finally, as shown in Table 1, a total of 20 studies (13-21,25-27,30-37) were found to meet the inclusion criteria for assessing the intracranial efficacy of ALK inhibitors and included in this analysis. In total, 929 out of 2,715 NSCLC patients had BMs at baseline when enrolled, in which 337 had measurable disease. Twenty studies involving three phase 1 trials (14,27,32), eight phase 2 trials (15-17,25,26,30,31,33), five phase 3 trials (18-21,36), one observational study (37), and three retrospective analysis (13,34,35) were included in the following analysis. Six assessed alectinib (16,17,30,31,36,37), five studies assessed ceritinib (14,15,18-20), four assessed crizotinib (13,21,34,35), two assessed brigatinib (25,26), one assessed lorlatinib (27), one assessed entrectinib (32), and one assessed ensartinib (33). All phase 3 studies were open-labeled, in which the control arms consisted of standard chemotherapy (18-21,36). ALK inhibitors were administrated as monotherapy in all included studies, different dose settings were found in five studies. Twenty studies were included in the analysis on tumor response, three studies on DCR, and two on PFS.
Full table
Meta-analysis results
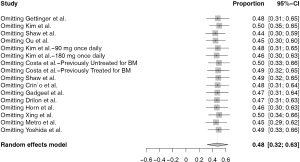
As shown in Figure 2A, for single-arm studies, fifteen studies included available data of iORR, the overall ORR was 48% (95% CI: 32–63%), in which the overall complete remission rate was 21% (95% CI: 12–32%) in six studies with available data (Figure 2B). High heterogeneity were seen in the meta-analysis of iORR and intracranial complete remission with I2 score of 92% (P<0.01) and 74% (P<0.01), respectively. Therefore, a random-effects model was used. Three studies include available data of iDCR (Figure 2C), the overall DCR was 65% (95% CI: 60–69%). The I2 score was 1% (P=0.40), indicating a low heterogeneity, and the fixed-effects model was conducted.
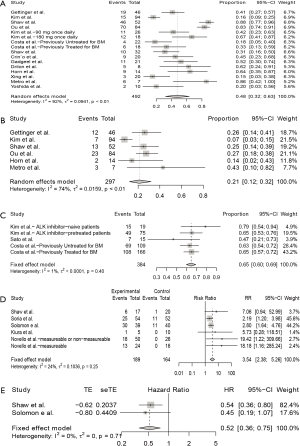
Five randomized studies assessed the intracranial efficacy of anti-ALK agents versus chemotherapy in patients with advanced NSCLC, all were 2-arm trials. The pooled RR for iORR was 3.54 (95% CI: 2.38–5.26) (Figure 2D). Since the iPFS was available in 2 studies, the pooled HR for iPFS was estimated in 2 studies, with a pooled HR for PFS of 0.52 (95% CI: 0.36–0.75; P=0.71) (Figure 2E). No heterogeneity was detected for iORR or iPFS, with I2 score of 0% (P=0.25) and 0% (P=0.71), respectively.
Subgroup analysis was performed to assess the influence of baseline BMs, previous treatment with ALK inhibitor, study type, and current ALK inhibitor on the intracranial ORR (Figure 3), revealing that iORR differed according to study type (Q=7.38, P=0.0250), and current ALK inhibitor treatment (Q=66.13, P<0.0001). The iORR observed in patients receiving alectinib was 79% (95% CI: 64–91%), in those treated with ceritinib was 45% (24–67%), in those treated with brigatinib was 48% (34–63%), and in those receiving crizotinib was 18% (13–24%).

Sensitivity analysis
We performed sensitivity analyses by sequentially removing each included study to assess the influence of individual study on the pooled proportion of iORR and iDCR in single-arm studies, and pooled RR for iORR as well as pooled HR for iPFS for randomized studies. The omission of any study did not have a significant effect on the results, suggesting that the results of this meta-analysis are statistically reliable (Figure S1).
Quality evaluation and publication bias
Quality of trials and risk of bias according to the Cochrane ‘Risk of bias’ are reported in Table 2. Publication bias was evaluated by funnel plot and Egger’s test. The funnel plot for the iORR in single-arm studies showed an even spread of studies on either side of the overall effect estimate line, as seen in Figure S2A. The results of Egger’s test also revealed no publication bias (t=−0.202, P=0.8426, Figure S2B).
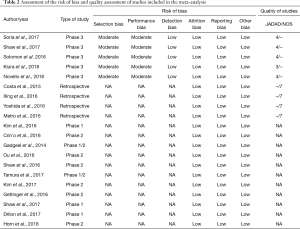
Full table

Only three studies were included to describe iDCR, and five studies were included to evaluate randomized studies. Since at least ten studies are required to be included to conduct a funnel plot, otherwise, the test power will be too low to assess the symmetry of the funnel plot. Therefore, we did not generate a funnel plot to assess publication bias for iDCR or randomized studies due to the limited number of studies included.
Discussion
Higher likelihood of development of CNS metastases in patients with ALK-rearrangement NSCLC indicates that new effective treatment options are in urgent need. In this meta-analysis, ALK inhibitors, especially second- or third-generation agents showed favorable intracranial activities in patients with ALK-rearranged NSCLC and BMs with an iORR of 48% in first- or further-line setting. This efficacy was not dependent by previous ALK inhibitor treatments or baseline BMs, and alectinib appeared to be most active in this setting. However the quality of evidence supporting these findings is low.
BMs is a major concern for ALK-positive NSCLC, the overall incidence of BMs in patients with ALK-positive NSCLC is high, with 35% to 50% of enrolled patients had stable or asymptomatic BMs (11,38). Also, the incidence of CNS metastases appears to increase with disease course, as many as 60% of crizotinib-treated patients progressed first in the CNS (13). Crizotinib has been considered to have lower efficacy in BMs due to its poor penetration across the BBB (0.0026) (39,40), however, a pooled retrospective analysis assessed data from patients enrolled in the profile 1005 trial and crizotinib-treated patients in the profile 1007 trial, showing that crizotinib was associated with a moderate iORR (18% to 33%) among patients with measurable BMs, and a DCR around 56–62% (13). Local treatment and the continuation of crizotinib has been a generally accepted strategy for ALK-positive NSCLC patients who develop BMs. Newer-generation ALK inhibitors have been developed to overcome the resistance to crizotinib with improved CNS activities. CNS antitumor activity has been described with ceritinib both in the crizotinib-resistant and frontline setting. The intracranial response rate with ceritinib was 45% (95% CI: 23.1–68.5%) for patients who previously progressed on crizotinib (15). In the phase III ASCEND-4 trial comparing first-line ceritinib with chemotherapy in 376 treatment-naive and ALK-positive NSCLC patients, 44 patients had measurable BMs at baseline. The intracranial response among those receiving ceritinib was 73% (95% CI: 49.8–89.3%) versus 27% (95% CI: 10.7–50.2%) among those in the chemotherapy group, and the median PFS was 10.7 versus 6.7 months (HR 0.70, 95% CI: 0.44–1.12) for those with BMs receiving ceritinib or chemotherapy, respectively (19). Different from crizotinib or ceritinib, preclinical studies demonstrated that alectinib is not a substrate of P-glycoprotein (P-gp), a key drug efflux pump typically expressed in the blood–brain barrier (41,42). Clinically, alectinib has demonstrated significant intracranial activity in the post-crizotinib settings, with an ORR of in crizotinib-naïve and 52–57% in crizotinib-resistant/intolerant patients (16,17). Alectinib also demonstrated improved intracranial activities to crizotinib, objective responses rate have been seen in 81% patients of treated with alectinib compared with 50% in crizotinib group (23). Moreover, there is evidence that alectinib has activity in patients who have developed progressive brain or leptomeningeal metastases on ceritinib or crizotinib (24). Brigatinib has been evaluated in a phase 1/2 trial and a randomized phase 2 trial (ALTA1) in advanced ALK-arrangement NSCLC, showing a favorable intracranial ORR around 50–67%, especially in those treated with higher-dose brigatinib (lead-in at 90 mg/d followed by 180 mg/d) (25,26). Lorlatinib is a third-generation, CNS-penetrating ALK/ROS1 inhibitor, its phase 1 trial displayed significant CNS penetrability of lorlatinib with a mean ratio of cerebrospinal fluid (CSF) to plasma concentrations of 0.75, and an intracranial response rate of 42% (95% CI: 20–67%) among 19 heavily treated, ALK-positive patients with measurable CNS disease (27). Its intracranial activities were further confirmed in a phase 2 study including six different expansion cohorts for ALK-positive patients based on prior treatment, the intracranial response rate of lorlatinib was 68% (95% CI: 50–82%) for crizotinib-treated patients, and 48% (95% CI: 37–59%) for patients previously treated with two or three ALK inhibitors (28). Promising intracranial activities were also seen in entrectinib and ensartinib in early studies (32,33).
In the current study, among 2,715 patients from 20 studies, a total of 929 (34.2%) patients have BM at baseline with or without previous treatment with ALK inhibitors, including 337 patients with measurable disease. We observed favorable intracranial activities of ALK inhibitors with an overall iORR of 48% (95% CI: 32–63%). Current anti-ALK treatment had significant impact on iORR, alectinib appeared to be more effective on controlling BM with an iORR of 79%, followed by brigatinib (48%), ceritinib (45%) and crizotinib (18%). Only five randomized study comparing ALK inhibitors with chemotherapy were included in this study, the pooled HR for PFS were 0.52 (95% CI: 0.36–0.75), and the pooled OR for ORR was 3.54 (95% CI: 2.38–5.26), indicating a significant improvement of ALK inhibitors in CNS efficacy.
Some limitations of our work need to be considered. First, it includes a very heterogeneous population due to the inconsistent designs of the included studies, so we cannot draw overall conclusions. In addition, we did not evaluate the impact of prior radiotherapy, which have already been established as standard treatment for CNS disease control. We also could not assess the CNS-related toxicities of ALK inhibitors. It should also be noted that most studies included were single-arm studies which provided low-quality supporting evidence, future studies based on evidence from randomized studies were needed. Finally, we have not taken into account parameters such as intracranial PFS, time to response or duration of response; these data would have improved the evaluation of the real benefit with ALK inhibitors.
Targeted therapies are increasingly incorporated into the multidisciplinary management of patients with BMs from oncogene-driven NSCLC. In conclusion, despite the low quality of supporting evidence, we can obtain a general overview in the current studies that ALK inhibitors are effective at the brain site regardless of previous anti-ALK treatment. Further studies are needed to compare the efficacy and safety between ALK inhibitors, as well as ALK inhibitors and local therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J Clin Oncol 1988;6:1474-80. [Crossref] [PubMed]
- Davey P. Brain metastases: treatment options to improve outcomes. CNS Drugs 2002;16:325-38. [Crossref] [PubMed]
- Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37:13-25. [Crossref] [PubMed]
- Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 2004;45:S253-7. [Crossref] [PubMed]
- Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123-9. [Crossref] [PubMed]
- Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 2015;16:e510-21. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Crinò L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Kiura K, Imamura F, Kagamu H, et al. Phase 3 study of ceritinib vs chemotherapy in ALK-rearranged NSCLC patients previously treated with chemotherapy and crizotinib (ASCEND-5): Japanese subset. Jpn J Clin Oncol 2018;48:367-75. [Crossref] [PubMed]
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol 2015;10:232-6. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Solomon BJ, Shaw A, Ou SH, et al. Phase 2 Study of lorlatinib in patients with advanced ALK+/ROS1+ non-small-cell lung cancer. Presented at: the IASLC 18th World Conference on Lung Cancer, October 15-18, Yokohama, Japan. Abstract 8573.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Tamura T, Kiura K, Seto T, et al. Three-Year Follow-Up of an Alectinib Phase I/II Study in ALK-Positive Non-Small-Cell Lung Cancer: AF-001JP. J Clin Oncol 2017;35:1515-21. [Crossref] [PubMed]
- Drilon A, Siena S, Ou SI, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov 2017;7:400-9. [Crossref] [PubMed]
- Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin Cancer Res 2018;24:2771-9. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Clinical impact of crizotinib on central nervous system progression in ALK-positive non-small lung cancer. Lung Cancer 2016;97:43-7. [Crossref] [PubMed]
- Xing P, Wang S, Hao X, et al. Clinical data from the real world: efficacy of Crizotinib in Chinese patients with advanced ALK-rearranged non-small cell lung cancer and brain metastases. Oncotarget 2016;7:84666-74. [Crossref] [PubMed]
- Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Metro G, Lunardi G, Bennati C, et al. Alectinib's activity against CNS metastases from ALK-positive non-small cell lung cancer: a single institution case series. J Neurooncol 2016;129:355-61. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Chun SG, Choe KS, Iyengar P, et al. Timmerman RD. Isolated central nervous system progression on Crizotinib: an Achilles heel of non-small cell lung cancer with EML4-ALK translocation? Cancer Biol Ther 2012;13:1376-83. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Tang SC, Nguyen LN, Sparidans RW, et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by co-administration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer 2014;134:1484-94. [Crossref] [PubMed]
- Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023-8. [Crossref] [PubMed]